This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Plant science research paves the way for deeper understanding of how the plant immune system functions

Researchers in the laboratory of Tessa Burch-Smith, Ph.D. at the Danforth Plant Science Center and the University of Tennessee, Knoxville, are conducting pioneering work to discover how plants transmit information, important molecules, and viruses between cells.
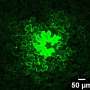
In a recent study they demonstrated how plasmodesmata (PD)—structures that connect neighboring cells in leaves and other organs—are controlled by deposition of callose (a carbohydrate polymer) when plants are responding to infection. Their research compared different methods to rigorously quantify callose accumulation around the microscopic PD channels and paves the way for a deeper understanding of how the plant immune system works.
Results of their study were recently published in "Comparing methods for detection and quantification of plasmodesmal callose in Nicotiana benthamiana leaves," in the journal Molecular Plant-Microbe Interactions..
Callose, a polymer made of glucose molecules, is essential for regulating intercellular trafficking via plasmodesmata (PD). Pathogens manipulate PD-localized proteins to enable intercellular trafficking by removing callose at PD or, conversely, by increasing callose accumulation at PD to limit intercellular trafficking during infection.
Plant defense hormones like salicylic acid regulate PD-localized proteins to control PD and intercellular trafficking during immune defense responses such as systemic acquired resistance.
Measuring callose deposition at PD in plants has emerged as a popular way to assess the likely trafficking of molecules between cells during plant immunity. Despite the popularity of this metric, there is no standard for how these measurements should be made.
First Author Amie Sankoh, Ph.D., and her undergraduate colleague, Joseph Adjei, compared three commonly used methods for identifying and quantifying PD callose by aniline blue staining and evaluated to determine the most effective in the leaf model. Both Amie and Joseph are Deaf and communicate primarily via American Sign Language.
Their results revealed that the most reliable method used aniline blue staining and fluorescent microscopy to measure callose deposition in fixed tissue. Manual or semi-automated workflows for image analysis were also compared and found to produce similar results, although the semi-automated workflow produced a wider distribution of data points.
"We were surprised at how different the reliability of the different methods for detecting callose could be. We think this work will greatly improve consistency in experiments across labs," said Dr. Sankoh.
This study relied on the Advanced Bioimaging Laboratory at the Danforth Center. The team plans to use the identified protocol and analysis to investigate how callose levels at PD change over the course of infection with various hormones. Such studies could identify important times at which PD could be manipulated to disrupt the infection process and prevent plant disease.
More information: Amie Fornah Sankoh et al, Comparing methods for detection and quantification of plasmodesmal callose in Nicotiana benthamiana leaves during defense responses, Molecular Plant-Microbe Interactions (2024). DOI: 10.1094/MPMI-09-23-0152-SC
Journal information: Molecular Plant-Microbe Interactions
Provided by Donald Danforth Plant Science Center