This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Findings suggest ILF3 may function as a reader of telomeric R-loops to help maintain telomere homeostasis
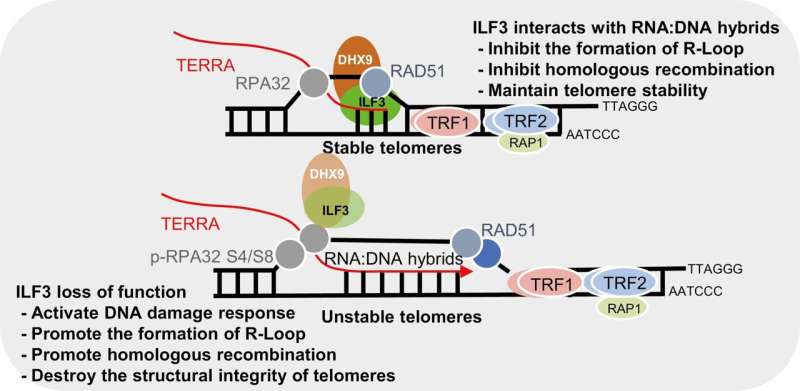
Dysregulated R-loops can cause stalled replication forks and telomere instability. However, how R-loops are recognized and regulated, is still not well understood, particularly at telomeres.
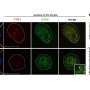
In a new study, researchers used proximity-dependent biotin identification (BioID) technology to identify the ILF3 interactome and discovered that ILF3 interacts with several DNA/RNA helicases, including DHX9. This interaction suggests that ILF3 may facilitate the resolution of telomeric R-loops, thereby preventing abnormal homologous recombination and maintaining telomere homeostasis.
The work, titled "ILF3 safeguards telomeres from aberrant homologous recombination as a telomeric R-loop reader," was published in Protein & Cell.
Key findings from the study include:
- ILF3 exhibits selective interact with telomeric R-loops, thereby safeguarding telomeres against aberrant homologous recombination.
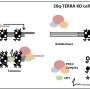
- ILF3 loss of function consequently elevates TERRA levels, triggering the accumulation of R-loops at telomeres. This accumulation induces DNA damage response (DDR) and telomere dysfunction, characterized by elevated TIFs, telomere fragility, and the presence of extra-chromosomal telomere fragments, which may in turn activate the ALT pathway.
- Additionally, mapping the ILF3 interactome revealed interactions with various DNA/RNA helicases, including DHX9, with a significant implication that ILF3 potentially assists in resolving telomeric R-loops through its interaction with DHX9.
- ILF3 potentially acts as a reader for telomeric R-loops, aiding in the prevention of aberrant homologous recombination and the maintenance of telomere homeostasis.
These results support that ILF3 interacts with telomeric RNA:DNA hybrid structures such as R-loops and promotes the resolution or inhibits excessive accumulation of R-loops through the RNA helicase DHX9.
This research provides new insights into the regulation of telomeric R-loops and the mechanisms that maintain telomere homeostasis, with implications for aging biology.
More information: Chuanle Wang et al, ILF3 safeguards telomeres from aberrant homologous recombination as a telomeric R-loop reader, Protein & Cell (2023). DOI: 10.1093/procel/pwad054
Provided by Frontiers Journals