This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Tracking and tracing members of the plant microbiome with DNA barcodes
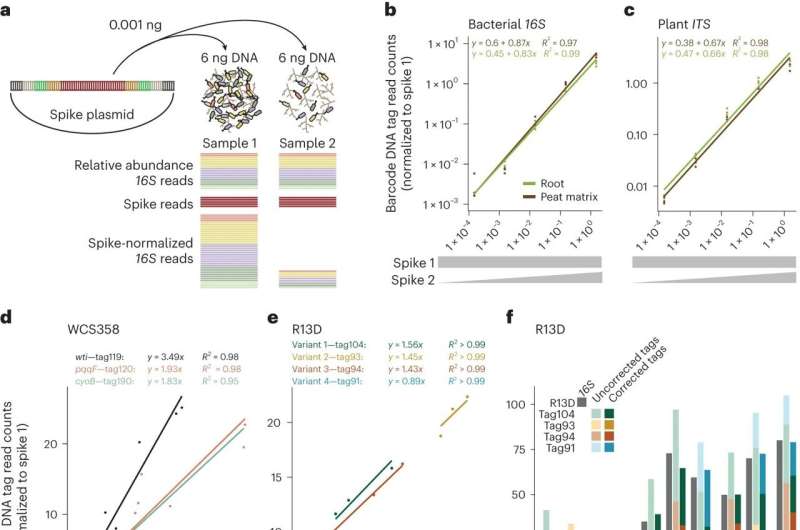
A research team led by Paul Schulze-Lefert from the Max Planck Institute for Plant Breeding Research in Cologne, Germany, developed a modular toolkit for tracking bacterial strains colonizing plant tissue in competition with other microbiome members. The study is now published in Nature Microbiology.
The enormous diversity of microorganisms that a healthy plant accommodates as a community in nature is referred to as the plant microbiome. In order to assess the composition of the microbiome, which is invisible to the naked eye, the DNA sequence of a universal microbial marker gene consisting of variable and conserved sequence segments is usually determined.
In this way, different microbial species in the microbiome can be distinguished from each other on the basis of polymorphic DNA marker sequences. However, beneficial activities of members of the microbiome for the plant host, such as the mobilization of mineral nutrients from the soil for uptake by the roots, are often only carried out by individual microbial strains within a bacterial species and depend on the presence of specialized microbial genes.
Hence, current DNA sequence-based microbiota profiling is insufficient to capture the true genetic diversity of the microbial community on the host.
To overcome this limitation, researchers at the Max Planck Institute have developed a modular toolkit that is used as a DNA barcode for bacterial strains. A DNA barcode is first inserted into the chromosome of a single strain of a microbiome member. In subsequent analyses of microbiome profiles on plants, the DNA barcode is regarded as a synthetic microbial marker gene.
In addition to the chromosomal DNA barcode, genetic building blocks for fluorescent proteins were also incorporated. The latter makes it possible to use highly sensitive fluorescence detectors to map where a barcoded bacterial strain colonizes plant tissue in competition with other microbiome members.
The researchers then carried out experiments with the plant growth-promoting bacterium Pseudomonas capeferrum, which colonizes the roots of the model plant Arabidopsis, as well as with variants of the bacterium that differ from the wild-type strain only in the absence of particular genes.
The corresponding Pseudomonas genes dampen the host plant's immune responses and thus enhance the bacterium's ability to colonize plant roots, which in turn increases its plant growth-promoting activity. The Pseudomonas bacteria labeled with DNA barcodes showed the expected differential ability to colonize Arabidopsis roots when germ-free plants were inoculated with individual strains.
What was surprising, however, was the appearance of qualitatively new characteristics of the Pseudomonas bacteria when combinations of the wild-type strain and its variants were inoculated onto the plant host together with a microbiome consortium of different bacteria assembled in the laboratory. In biology, this phenomenon is also referred to as an emergent property or system property.
The use of DNA barcodes, therefore, not only confirmed previous results but also revealed new activities of the bacterial genes that could not have been identified using conventional methods. The modular DNA barcoding toolkit can now be used for microbiome research to investigate the contribution of individual microbial genes in the context of microbial communities not only in plants but also in microbiome studies of animals.
More information: Jana Ordon et al, Chromosomal barcodes for simultaneous tracking of near-isogenic bacterial strains in plant microbiota, Nature Microbiology (2024). DOI: 10.1038/s41564-024-01619-8
Journal information: Nature Microbiology
Provided by Max Planck Society





















