This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Research team designs small-scale 'chemical nose'
A living organism's nose is essentially a biological molecule detector that sends neurological signals to the brain, which then decodes a particular scent. Human noses, with six million olfactory receptors, can distinguish more than one trillion scents, while some canine noses possess up to 300 million receptors, which provide enhanced sensitivity in parts per trillion.
"Electronic noses" are electronic devices that can "sniff" and identify vaporized odors and flavors. Typically connected to a significant amount of laboratory equipment, these synthetic noses are not readily portable, motivating researchers to devise new, transportable sensors that can identify a broad range of chemicals.
Researchers at the University of Pittsburgh Swanson School of Engineering have furthered that potential by designing a small-scale system that forms three-dimensional patterns, which serve as chemical "fingerprints" that allow chemicals in solutions to be identified. Principal investigator is Anna C. Balazs, Distinguished Professor of Chemical Engineering, with lead author and postdoc Moslem Moradi, and postdoc Oleg E. Shklyaev. The work appears in Proceedings of the National Academy of Sciences.
"Catalysts are highly selective; only certain reactants can trigger a particular catalytic reaction. Due to this selectivity, catalysts in a solution can reveal the identities of the reactants. If the right reactants are added to the fluid, then the resulting reaction generates the spontaneous flow of the fluid; the flow, in turn, can bend and shape flexible objects immersed in the solution," Balazs explained.
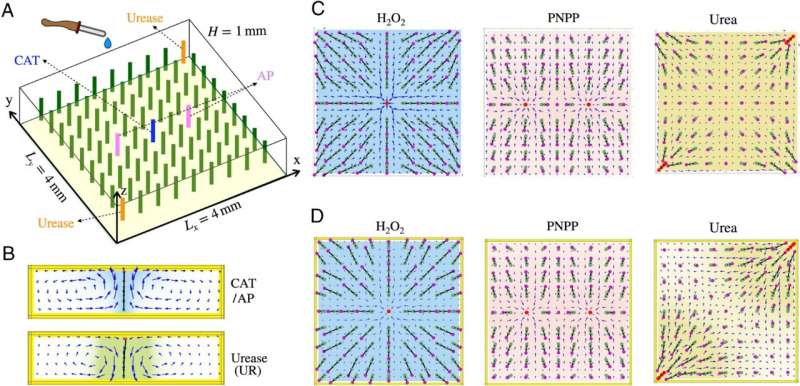
"If flexible posts are tethered to the base of a fluid-filled chamber and coated with specific enzymes, then the added reactants will force the posts to bend in different directions and form distinct visual patterns.
"What is amazing is that each reactant, or combination of reactants, produces a separate pattern. In effect, the chemicals leave a distinctive 'fingerprint,' which allows us to identify the chemical composition of the solution."
In the simulation, Moradi constructed a chamber four millimeters square and one millimeter in height, with 81 flexible posts. Only a few posts at particular locations were coated with one of three types of enzymes.
"If we examine specific reactions, we can discern the shapes they contribute to the overall pattern. Consequently, we can control the patterns and tune their appearance." Moradi said. "Moreover, if the reactants are added one at time, we can form a chemical kaleidoscope as one pattern smoothly morphs into another when the previous reactants are consumed by the reaction and a new reactant is added to the solution."
Shklyaev added that these results are notable because the posts are similar to electronic nodes. "The posts are like on-off switches and move in a specific direction regulated by the flow," he said, "and the patterns reveal the chemical fingerprints. The chemistry happens on the nanoscale, and we observe at the millimeter-scale visible patterns formed by the post, which can reflect light and thus could be detected by the naked eye."
Concurrently, the findings highlight a means of directing the flow in the chamber without building new walls for each application, potentially broadening the utility of a given fluidic device.
"Our tests used three different enzymes that allows us to generate multiple different patterns in response to only three different chemicals. Since each of the 81 posts could potentially be coated with a different enzyme, the total number of possible patterns increases exponentially with the number of the posts. On a conceptual level, the patterns are an analog of the electrochemical responses the brain makes to identify odors or scents."
Balazs said, "Since each reactant leaves a specific fingerprint, we can form a database of patterns. We can use this database to detect a hazardous chemical or waterborne toxin by comparing the generated pattern with others in the database to identify a match. Our system lays the groundwork for a simple, portable toolkit that allows you to add the chemical into a chamber and the resulting visual pattern identifies the substance. It's a beautiful yet simple chemical nose."
More information: Moslem Moradi et al, Integrating chemistry, fluid flow, and mechanics to drive spontaneous formation of three-dimensional (3D) patterns in anchored microstructures, Proceedings of the National Academy of Sciences (2024). DOI: 10.1073/pnas.2319777121
Provided by University of Pittsburgh