This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Stronger than nature: Optimized radicals as potential novel catalysts
Nature uses enzymes for various metabolic processes. These biological catalysts are extremely efficient. Biomimetic catalysts based on inexpensive starting materials from the laboratory that can reproduce the efficiency of the natural enzymes and can function at ambient conditions are therefore of great interest to research and industry.
In a project led by the Institute of Chemistry at the Humboldt-Universität zu Berlin (HU), researchers have been investigating a special group of biological catalysts known as oxidases. These enzymes catalyze various oxidation reactions, where electrons are released from one substance and absorbed by another. Small, highly reactive particles, so-called radicals, often play an important role in these processes.
Oxidizing capacity improved by binding to iron
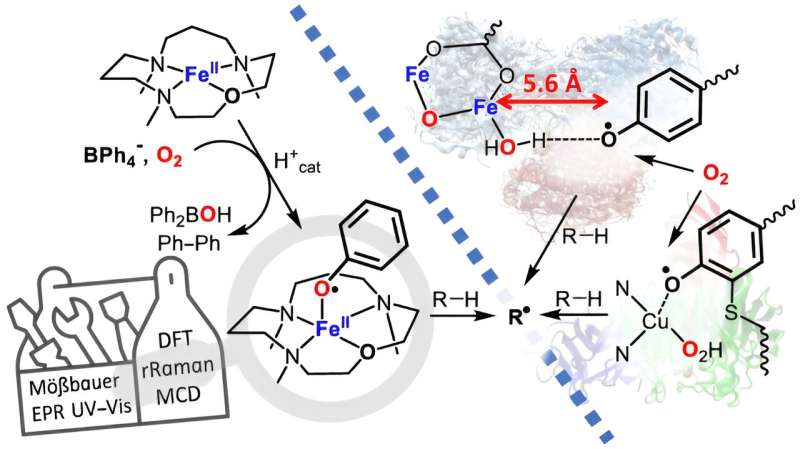
The enzyme of present interest is galactose oxidase found in many types of fungi, where a phenoxyl radical is used as the oxidant. The team led by HU researcher Kallol Ray has now found a way to utilize the phenoxyl radical in the laboratory in such a way that the oxidation capacity can be increased significantly.
In the naturally occurring enzyme, the phenoxyl radical is stabilized by a sulfur atom, which limits its oxidation capacity. The researchers have now improved the oxidation ability by attaching an unmodified phenoxyl radical to iron and have chemically characterized this iron-phenoxyl radical for the first time. Ray's team collaborated with colleagues from the Technical University of Berlin and the University of Michigan, U.S., on this project.
The work is published in the journal Nature Chemistry.
First description of the iron phenoxyl radical—important for research and industry
"We expect that our work will be the starting point for more targeted efforts to utilize the iron-phenoxyl radical interaction for various biochemical reactions," says Ray. "This can support the development of novel catalysts needed for alternative energy technologies and other biotechnological applications."
The research results of Ray and his team are of great importance for both research and application, as the reaction catalyzed by galactose oxidase (oxidation of a primary alcohol to the corresponding aldehyde) is one of the most important and most widely used chemical reactions in organic synthesis.
The findings could also be used in industry to convert the climate-damaging gas methane into liquid methanol. Unlike methane, which is a volatile gas and therefore difficult to handle, methanol is easy to transport and can be used as a synthetic fuel. Currently, a great deal of energy is required to convert methane into methanol. The chemical reaction takes place only at high temperatures (> 500 degree Celsius) and under high pressure. Biomimetic catalysts could significantly reduce this energy input.
"In this project, it was exciting to see the unexpected structural and functional resemblance of our synthetic system to natural enzymes," says Dustin Kass, a Ph.D. student in Kallol Ray's research group and the lead author of this study.
More information: Dustin Kass et al, Trapping of a phenoxyl radical at a non-haem high-spin iron(II) centre, Nature Chemistry (2024). DOI: 10.1038/s41557-023-01405-9
Provided by Humboldt University of Berlin