A radical approach to methane oxidation into methanol
Free radicals don't get the best press. However, while they are known as harmful oxidants in the body, these ultra-reactive chemicals are indispensable in the lab. Radical reactions play a role in key technologies such as pollutant removal and water-splitting.
Now, researchers at Osaka University have used radicals to transform a greenhouse gas, methane, into useful chemicals. Driven by light, this environment-friendly process achieves a goal that remained elusive for decades.
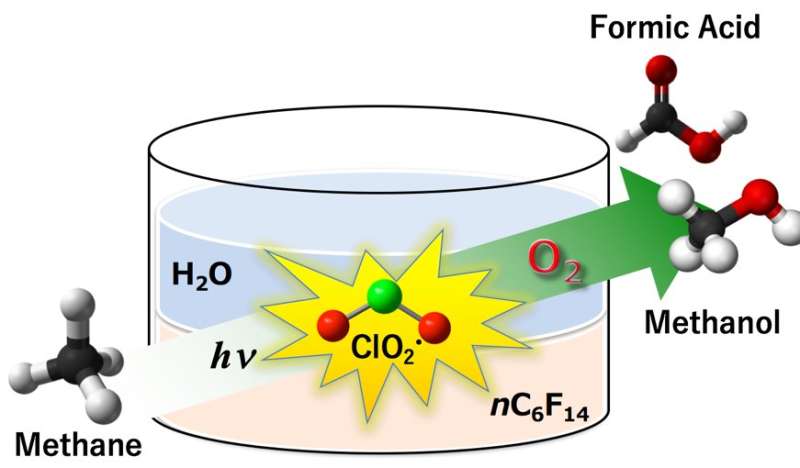
Methane (CH4) is related to methanol and formic acid, which are needed in huge quantities by the chemical industry. Bacteria can oxidize CH4 to methanol almost effortlessly using natural enzymes. The same transformation in the lab, however, chemically requires high temperature, high pressure and expensive reagents to cleave the extremely strong C—H bonds. As reported recently, the new process uses powerful chlorine radicals to activate those bonds. This allows the reaction to occur at room temperature, under lamp light, with simple oxygen as the oxidizing agent.
Free radicals are chemicals with unpaired electrons—their rampant reactivity comes from the urgent need for the lone electrons to find partners in another molecule. In the Osaka process, chlorite dioxide (ClO2•) is activated under the photoirradiation to give chlorine radicals (Cl•) and singlet oxygen. The highly reactive radical, Cl•, then abstracts a hydrogen atom of CH4 to give methyl radicals, CH3•, which in turn react with oxygen to produce valuable methanol and formic acid. This apparently simple process, however, relies on a subtle design twist.
"Methane activation by radical species has been tried before," study a lead author Prof. Kei Ohkubo says. "However, CH3• intermediates tend to react with the hydrocarbon organic solvent giving the deactivation of reactive the radical intermediates. This doesn't occur in water, but unfortunately methane barely dissolves in water." The researchers found a neat way around this: two solvents in a single system, one for each step of the process. The initial ClO2• formation occurs in a water phase, where sodium chlorite is soluble. Then, ClO2• transfers to a perfluorohexane (PFH) phase, where methane and O2 dissolve to react with them.
"PFH is ideal for the second step: it dissolves methane, but doesn't react with CH3• radicals," explains Ohkubo. "This creates a space for the oxidation of CH3•, giving the desired products. Then, after methanol and formic acid are formed, they cross the solvent boundary in the opposite direction, into the water phase. Here they are protected against further oxidation into unwanted CO or CO2 as greenhouse gasses."
The complete process is impressively efficient, converting over 99% of methane into the target products, without the need for high temperature or pressure.
"This is the first successful use of oxygen in the air to oxidize methane under ambient conditions," Ohkubo says. "Energy-intensive methods for chemical production must be phased out—we urgently need smart solutions to process raw materials in a gentle, environmentally benign way. Our study shows how this can be done for methane. The two-phase solvent concept, where unstable intermediates are protected by a solvent such as PFH, could potentially be extended throughout industry."
More information: Kei Ohkubo et al. Light-driven C-H Oxygenation of Methane into Methanol and Formic Acid by Molecular Oxygen Using Perfluorinated Solvent, Angewandte Chemie International Edition (2017). DOI: 10.1002/anie.201710945
Journal information: Angewandte Chemie International Edition
Provided by Osaka University