This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists reveal role of neighboring adsorbates and quantum tunneling in surface diffusion of hydrogen atoms
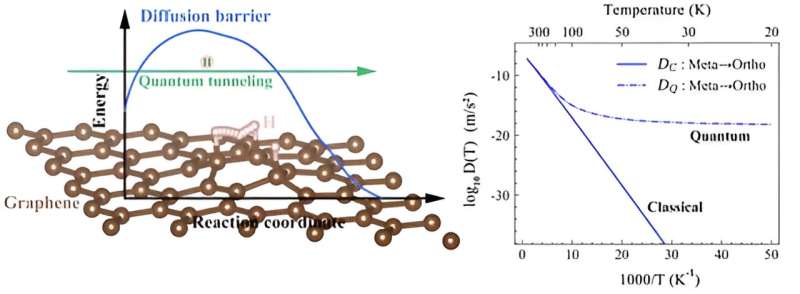
A research group led by Prof. Yang Yong from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences has revealed the role of neighboring adsorbate and quantum tunneling in the diffusion of hydrogen on a graphene surface, which opens a possible way for ultrahigh precision measurement based on atomic systems, in particular, exploring the existence of a minimum length.
The results are published in The Journal of Physical Chemistry C.
Hydrogen, the lightest element, exhibits quantum motion known as the nuclear quantum effect in its dynamical processes. The study conducted by Yang's team demonstrates the crucial role of quantum tunneling in the activation of hydrogen dissociation and diffusion processes on copper surfaces. On graphene surfaces, hydrogen shows different aggregation states depending on the coverage.
To study the diffusion of hydrogen in various aggregation states on a graphene surface, the researchers used first-principles calculations together with the transfer matrix method. They examined the quantum tunneling effects on hydrogen diffusion by calculating transmission probabilities, rate constants, and diffusion coefficients, considering hydrogen atoms as classical and quantum particles, respectively.
The adsorption of hydrogen atoms on neighboring adsorption sites will significantly change the kinetic properties of the diffusing hydrogen atoms on a graphene surface. The interaction between neighboring hydrogen atoms was found to be a key factor leading to the variation of the diffusion barrier height.
In the diffusion of hydrogen atoms in different aggregation states, the comparison of the diffusion probability, reaction rate constant and diffusion coefficient of hydrogen as classical particle and quantum particles show that quantum tunneling plays a key role in the diffusion at room temperature and below. Even in the higher temperature region (around 600 K), the contribution is still not negligible.
"Our results provide new insights into understanding the diffusion dynamics of hydrogen atoms on the graphene surface," said Prof. Yang.
More information: Yangwu Tong et al, Hydrogen Diffusion on Graphene Surface: The Effects of Neighboring Adsorbate and Quantum Tunneling, The Journal of Physical Chemistry C (2024). DOI: 10.1021/acs.jpcc.3c05315
Journal information: Journal of Physical Chemistry C
Provided by Chinese Academy of Sciences