This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Surface-lattice-confinement effect accelerates hydrogen spillover
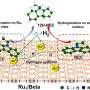
Hydrogen spillover is the dynamic migration of surface-adsorbed hydrogen species from hydrogen-rich sites to hydrogen-poor sites. It plays an important role in many H-involving reaction processes.
In order to enhance the catalytic performance of H-involving reactions, it is important to understand the detailed mechanism of hydrogen spillover and learn how hydrogen transfers and what factors control hydrogen conductivity on solid surfaces.
Recently, a research team led by Prof. Mu Rentao and Prof. Fu Qiang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) directly observed the acceleration of hydrogen spillover via a surface-lattice-confinement effect.
This work was published in Nature Communications on Feb. 4.
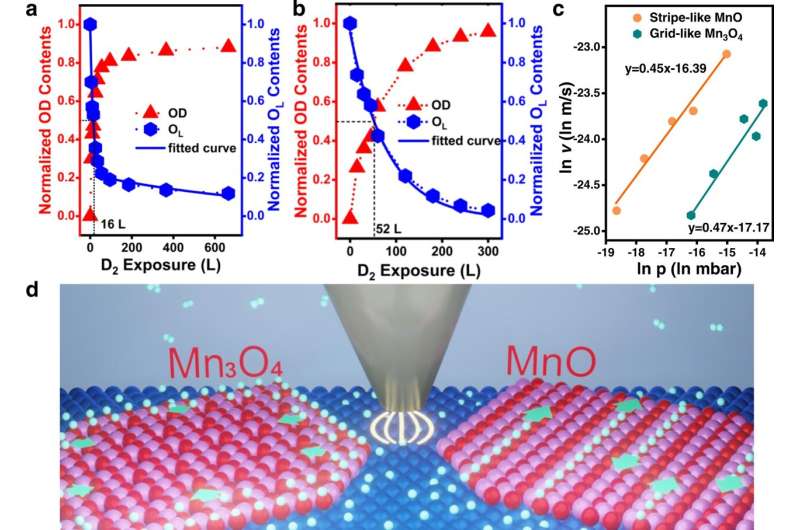
The researchers constructed stripe-like MnO(001) and grid-like Mn3O4(001) monolayers on a Pt(111) substrate, and investigated their hydrogen spillover.
They found that hydrogen species from Pt diffused unidirectionally along the stripes on MnO(001), whereas it exhibited an isotropic pathway on Mn3O4(001).
Moreover, by using dynamic surface imaging in an H2 atmosphere, they revealed that hydrogen diffused four times more rapidly on MnO than was the case on Mn3O4, which was promoted by a one-dimension surface-lattice-confinement effect.
Theoretical calculations indicated that a uniform and medium O-O distance favored hydrogen diffusion while low-coordinate surface O atom inhibited it.
"Our study illustrates the surface-lattice-confinement effect of oxide catalysts on hydrogen spillover and provides a promising route to improve the hydrogen spillover efficiency," said Prof. Fu.
More information: Yijing Liu et al, Direct observation of accelerating hydrogen spillover via surface-lattice-confinement effect, Nature Communications (2023). DOI: 10.1038/s41467-023-36044-8
Journal information: Nature Communications
Provided by Chinese Academy of Sciences