This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How signaling proteins get to the mitochondrial surface
Mitochondria are organelles that are known for providing the energy currency that fuels chemical reactions within cells, but they are also involved in other important processes vital for cell health including the innate immune response to pathogens like viruses, programmed cell death, and communication with the rest of the cell—processes that all play a role in health and disease. The signaling proteins that serve as the mitochondria's interface with the rest of the cell are key players in these processes.
The outer surface of the mitochondria, known as the outer mitochondrial membrane (OMM), is thereby a focal point of control for mitochondrial dynamics and cell health and is filled with signaling proteins. The cell can change the protein composition of the OMM to tailor the function of individual mitochondria, but pathogens can also manipulate the OMM landscape to their benefit. Additionally, imbalances in the OMM protein composition have been linked to diseases such as cancer and neurodegenerative diseases including Parkinson's and Alzheimer's.
The better that researchers understand the dynamics of these OMM proteins, the more insights they may gain into the roles that they play in mitochondrial functions and their relevance to health and disease. That is why Whitehead Institute Member Jonathan Weissman, also a professor of biology at the Massachusetts Institute of Technology and a Howard Hughes Medical Institute investigator; Alina Guna, a joint postdoc in Weissman's lab and California Institute of Technology Assistant Professor Rebecca Voorhees' lab; and Gayathri Muthukumar, a graduate student in Weissman's lab, set out to learn more about how one of the major subsets of OMM proteins—the alpha-helical proteins—is created and regulated.
The researchers' detailed model of the different pathways and molecules involved in managing this portion of the OMM protein landscape was published in the journal Molecular Cell on February 29.
"The OMM alpha-helical proteins are a large and varied class, and that led us to the question of how the cell coordinates biosynthesis of these many different proteins," Muthukumar says. "Now that we have a broader, more complete sense of the specific molecular players involved and how the pathways work, that allows us to get a better sense of the OMM as a signaling platform, and how that gets manipulated under disease conditions."
A difficult bunch of proteins to get in position
The proteins that the researchers looked at are all trans-membrane proteins, meaning that they are inserted into and cross the outer membrane. They are also alpha-helical, which means they have a coil or helix shape in their trans-membrane domains, the parts of the protein that cross through the membrane. Some of the proteins only cross the membrane once, with one end outside of the mitochondrion and one end inside. Others get folded so that they weave in and out of the membrane multiple times.
Transmembrane proteins are challenging for the cell to assemble correctly. The basic parts of the protein are formed by machinery in the main area of the cell, and then they must be delivered to the OMM. The trans-membrane domains of the proteins are stable once inserted, but while the nascent proteins are in the main body of the cell, these domains are unstable and prone to clumping together.
Often, these proteins require so-called chaperones that pair with and protect them on the journey to the OMM, or else they will be degraded or clump together. Clumping not only prevents the proteins from getting to the OMM and doing their jobs there, but the errant clumps can themselves create problems for the cell. Another challenge is that transmembrane proteins are prone to errors in folding, which disrupts their function and can contribute to disease.
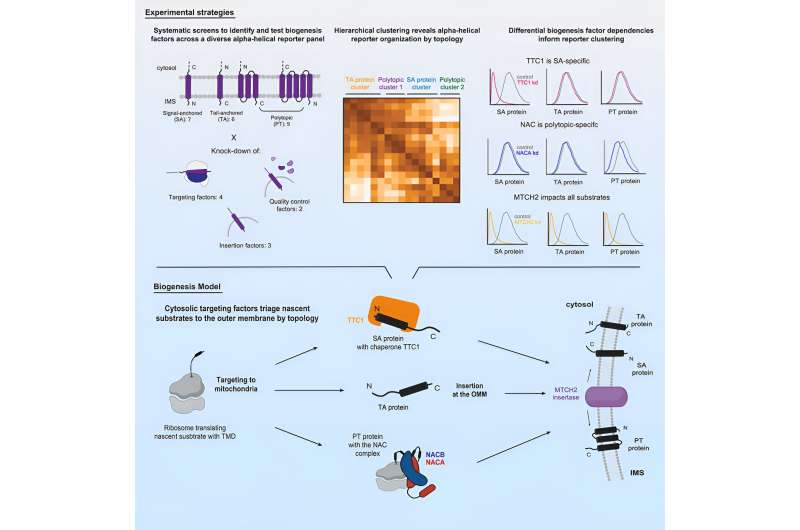
Weissman and colleagues thereby wanted to understand what mechanisms the cell uses to ensure that these proteins get safely delivered to and inserted into the OMM, or that the proteins get destroyed if there are errors in their synthesis. The researchers performed large-scale, systematic genetic screens using the CRISPR interference (CRISPRi) screening approach developed by Weissman and collaborators to look for the molecules that are required to get a variety of OMM proteins in place.
Casting a wide net reveals a variety of molecular players
The researchers, led by first author of the study Muthukumar, found that the proteins follow different pathways to the OMM depending largely on two factors: whether they cross the membrane once or multiple times, and which end of the protein faces the outside versus the inside of the mitochondrion.
The researchers ended up finding distinct pathways for three categories of proteins: signal-anchored proteins, which go through the membrane once and have the start of the protein facing inside; tail-anchored proteins, which go through the membrane once and have their start facing outside; and polytopic proteins, which go through the membrane multiple times (and appear to rely on the same pathway regardless of signal and tail orientation).
The researchers used their screening approach to test what molecules are essential for the proper delivery, insertion, and quality control or degradation of each category of OMM protein. They found that tail-anchored proteins do not, as far as they could tell, require any sort of chaperone to get to the OMM. Signal-anchored proteins do require a novel chaperone called TTC1 whose role in the cell was previously unknown.
The researchers performed further experiments to characterize TTC1 and gain insights into how it interacts with the OMM proteins. They used an AI system called AlphaFold to generate AI-derived models of TTC1 and with these models identified a new interface through which TTC1 keeps the trans-membrane domains stable in the main body of the cell. Finally, the researchers found that polytopic proteins are chaperoned by the NAC complex, a protein complex that had been known as a chaperone, but only for a specific class of proteins; this finding expands its role.
The majority of the proteins' pathways then converge on a single path for insertion into the OMM. They are predominantly guided by the insertase (insertion-facilitating molecule) MTCH2, recently identified by the Weissman and Voorhes labs.
Additionally, the researchers gained some insights into the molecules that perform quality control on OMM proteins, degrading them when there is an issue: they found that some tail-anchor proteins can be degraded in the main body of the cell by the molecule UBQLN1, and all three types can be degraded once in the OMM by the molecule MARCHF5. The researchers note that quality control was not the focus of their study, and that follow up studies are needed to learn more about quality control mechanisms.
Altogether, these findings enabled the researchers to make a detailed map of the pathways by which alpha-helical proteins are delivered to and inserted into the OMM, as well as to understand some of the ways in which they are regulated for quality control. The scope of the findings was only possible thanks to the large-scale genetic screening approaches that Weissman and others have developed in recent years.
"By looking at multiple different types of proteins, and multiple proteins of each type, and building a comprehensive parts list for their synthesis, we're able to pull out both the general principles for the protein types and the idiosyncrasies of each particular protein within that," Weissman says. "I think this project demonstrates how you can use systematic approaches and classic cell biology to open up a problem in a major way in a single paper."
These approaches allowed the researchers to collect a massive amount of data, which they intend to continue analyzing to make more discoveries about OMM proteins and the molecules that interact with them. Furthermore, the researchers hope that their current findings can provide new insights into the dynamics of the OMM as a signaling platform for the cell, a hub that is involved in many key processes in health and disease.
Understanding the pathways that control the OMM protein composition may help researchers discover how changes to this composition affect mitochondrial function and dysfunction. The findings may even ultimately enable researchers to alter the OMM protein landscape, which could lead to therapies for OMM-related diseases from cancer to neurodegeneration to viral infection.
"Because of their importance, imbalances in the OMM proteins have been linked to a variety of diseases," Guna says. "Therefore, how these proteins get made is not only a fundamental problem in cell biology, but also has wide-ranging implications. If we can understand how these proteins are made, it gives us the ability to intervene in healthy or disease states to change the composition of the OMM proteome and potentially steer a cell towards or away from a particular fate."
More information: Gayathri Muthukumar et al, Triaging of α-helical proteins to the mitochondrial outer membrane by distinct chaperone machinery based on substrate topology, Molecular Cell (2024). DOI: 10.1016/j.molcel.2024.01.028
Journal information: Molecular Cell
Provided by Whitehead Institute for Biomedical Research