This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Liquid crystal nanoparticles could supercharge antibiotics for cystic fibrosis
Cystic fibrosis is the most common, life-limiting genetic condition in Australia. It affects the lungs, digestive system, and reproductive system, producing excess mucus, infections, and blockages.
Now, University of South Australia researchers are advancing the development of liquid crystal nanoparticle-formulated antibiotics to more accurately target and eliminate difficult-to-cure lung infections in people with cystic fibrosis.
The study will use a patent-protected platform technology, invented by UniSA's Center for Pharmaceutical Innovation to establish new therapies for cystic fibrosis sufferers. UniSA will also work with the Cystic Fibrosis Airways Research Group at the Women's and Children's Hospital to advance the platform.
In Australia more than 3,600 people live with cystic fibrosis with one in every 2,500 babies born with the disease.
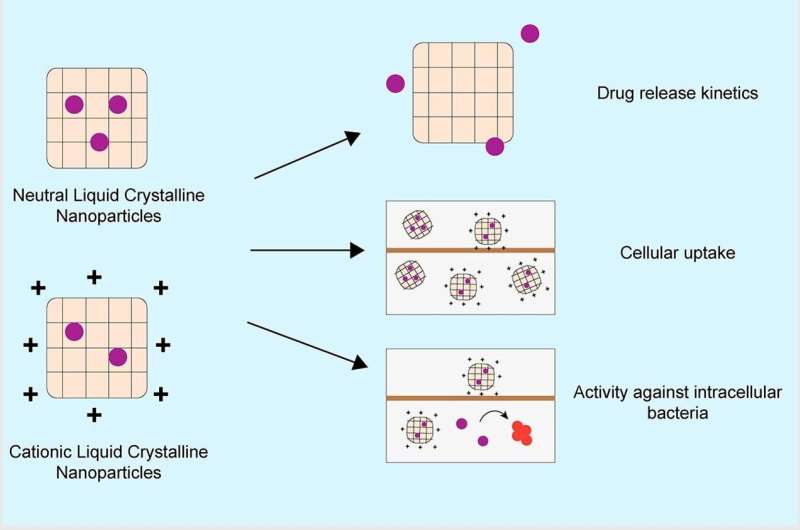
Lead investigator UniSA's Professor Clive Prestidge says that liquid crystal nanoparticles present a unique encapsulation and delivery system to improve the efficacy of antibiotics and overcome issues of antimicrobial resistance.
"When a person has cystic fibrosis, their body produces a sticky, thick mucus in the lungs which is prone to infection," Prof Prestidge says.
"Bacterial lung infections often require antibiotics, but with frequent infections and regular ineffective antibiotic use, bacteria are becoming resistant to treatments; the looming antimicrobial resistance (AMR) pandemic is a major threat to human health.
"When there is infection and blockages in the lungs, it's particularly hard for traditional antibiotics to reach their target. That's where liquid crystal nanoparticles can help.
"By overcoming the processes that cause drug resistance and uncontrollable infection, this unique delivery approach can better target sites in the body where conventional antibiotic therapies cannot penetrate."
Postdoctoral researcher and team member, UniSA's Dr. Santhni Subramaniam says preclinical studies have already demonstrated excellent performance against such infections.
"Whether it's bacteria in urinary tract infections, bone infections or bacterial biofilms found in tissue wounds, sinuses, and lung infections, preclinical trials of liquid crystal nanoparticles have delivered very positive results," Dr. Subramaniam says.
"We are now positioned to advance a nebulization approach for direct lung delivery.
"This is an exciting new technology that we hope will deliver significant improvement in people struggling with cystic fibrosis and other lung infections."
Previous research on the topic has been published in the International Journal of Pharmaceutics and the journal Small.
More information: Santhni Subramaniam et al, Liquid crystalline lipid nanoparticles improve the antibacterial activity of tobramycin and vancomycin against intracellular Pseudomonas aeruginosa and Staphylococcus aureus, International Journal of Pharmaceutics (2023). DOI: 10.1016/j.ijpharm.2023.122927
Chelsea R. Thorn et al, Tobramycin Liquid Crystal Nanoparticles Eradicate Cystic Fibrosis‐Related Pseudomonas aeruginosa Biofilms, Small (2021). DOI: 10.1002/smll.202100531
Journal information: Small
Provided by University of South Australia