This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Electronic perturbation-promoted interfacial pathway for facile C–H dissociation
Pt-based catalysts have been extensively employed in catalytic propane dehydrogenation (PDH) processes, playing a crucial role in propylene production. However, monometallic Pt catalysts often exhibit inferior propylene selectivity due to the hydrogenolysis, rapid deactivation from coke deposits, and nanoparticle sintering. To address these challenges, various metals (Sn, Zn, Ga, Co, etc.) have been introduced to enhance the selectivity and stability of Pt-based catalysts.
Typically, the formation of metal alloys, responsible for modulating the geometric and electronic structure of Pt, is considered a primary factor contributing to improved performance. Nevertheless, this modification tends to suppress the ability of Pt to activate C-H bonds, a critical aspect in dehydrogenation reactions.
The activation of strong C-H bonds within alkane molecules demands a significant energy input, resulting in relatively high barriers for the dehydrogenation process. To further enhance the catalytic efficiency of Pt-based catalysts in PDH, a more intricate design of active centers with a robust ability to activate propane and a high selectivity for propylene remains a necessary endeavor.
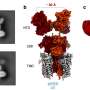
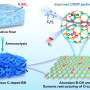
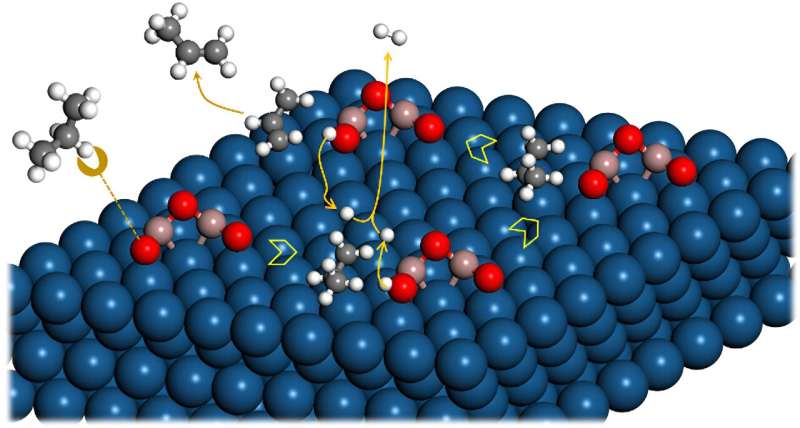
Recently, a research team led by Prof. Yong Wang from Zhejiang University, China, reported a remarkably efficient interfacial pathway for facile C-H bond scission at the Pt-GaOx interface by inducing O sites through electronic disturbance of Pt. Ga2O3 species on Pt (111) act as a bridge, enabling easy dehydrogenation.
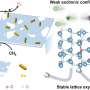
This process involves the ionization of the propane molecule to a proton and a mild alkyl shift, followed by smooth H spillover at high temperatures. Importantly, this mechanism differs significantly from the radical mechanism observed on monometallic and/or alloyed Pt surfaces.
The results have been published in the Chinese Journal of Catalysis.
Density functional theory calculations reveal that the oxygen sites of Ga2O3 species on the Pt surface exhibit higher-lying O 2p states and a considerable density of states around the Fermi level due to the electronic disturbance of the underlying Pt.
Consequently, these sites display a pronounced affinity for H and an exceptionally low energy barrier (less than 0.30 eV) for C-H dissociation. Furthermore, the Ga oxide component also contributes to the modification of the geometric structure of Pt nanoparticles. This modification leads to a reduction in ensemble size, promoting the selective production of propylene.
The designed Pt/Ga-Al2O3 catalysts demonstrate their superior performance in the PDH reaction compared to benchmark PtSn/Al2O3 catalysts.
More information: Zhe Wang et al, Electronic perturbation-promoted interfacial pathway for facile C–H dissociation, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64575-9
Provided by Chinese Academy of Sciences