This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Strong coordination interaction identified as being favorable for promoting ethanol dehydrogenation
Butadiene, one of the most important light olefins in the petrochemical industry with a global production capacity of 18 million metric tons per year, is currently produced through the extractive distillation of C4 fractions from naphtha steam cracking processes or dehydrogenation of C4 hydrocarbons, both of which involve extensive energy consumption and significant emission of CO2.
The increasing market demand has raised much interest in on-purpose production of these feedstocks. Thus, an alternative route to directly produce butadiene using any sustainable feedstocks is desirable and prospective.
Ethanol, as a carbon-neutral resource, can in principle undergo C-C bond formation to yield butadiene sustainably. Traditional catalytic systems have been classified into two categories: group 4 and 5 transition metals, and MgO-SiO2.
Among reported catalysts, Zn-Y/Beta is particularly noteworthy owing to its good selectivity for butadiene (>60%). Although great efforts were put into this direction, the steady production of butadiene from ethanol under mild reaction conditions remains an unsolved challenge, generally leading to quick deactivation. Another difficulty for butadiene formation is that the dehydration often competes with dehydrogenation reaction over Lewis acidic catalyst.
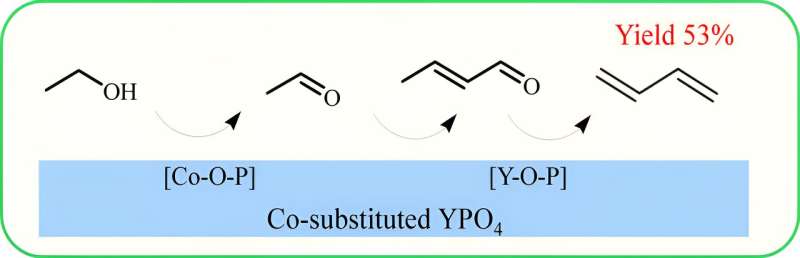
A research team led by Prof. An-Hui Lu from Dalian University of Technology, China, has reported the first cobalt-doped yttrium phosphate (Co-YPO4) catalyst, for preferential activation of ethanol to form acetaldehyde and subsequent C-C coupling and dehydration to butadiene. The catalyst exhibited 68.5% selectivity to butadiene in an ethanol conversion of 78.2% at 350 °C, and thereby close to 61% yield to total olefin (butadiene and ethene). The work is published in the Chinese Journal of Catalysis.
Combined with various in situ characterizations, a strong coordination interaction between Co2+ sites and the phosphate group on YPO4 has been identified as being favorable for improving ethanol dehydrogenation performance.
The YPO4 surface exposed the Y3+ site, which can effectively catalyze the C-C coupling reaction. Through the combination of the Co and Y species in one catalyst, i.e. Co-YPO4, the synergistic effect of the bifunctional sites could be achieved.
More information: Bai-Chuan Zhou et al, PO43– coordinated Co2+ species on yttrium phosphate boosting the valorization of ethanol to butadiene, Chinese Journal of Catalysis (2024). DOI: 10.1016/S1872-2067(23)64567-X
Provided by Chinese Academy of Sciences