This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Structural and biochemical basis of methylmalonate semialdehyde dehydrogenase ALDH6A1
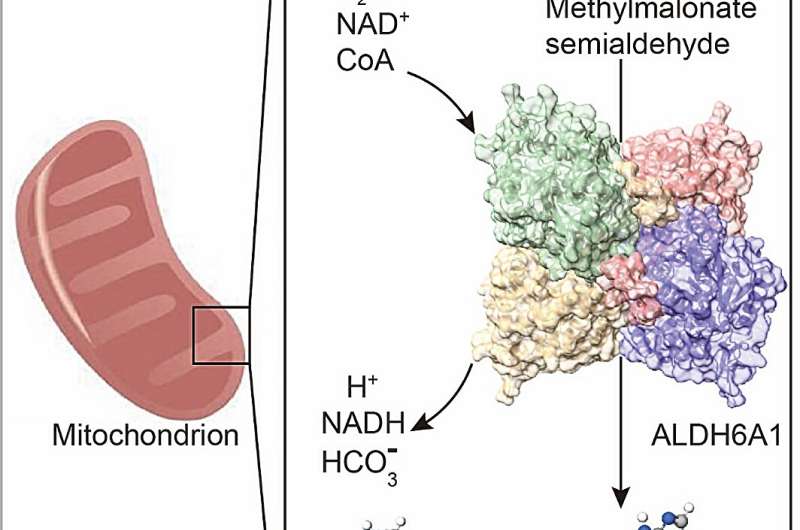
ALDH6A1, a member of the ALDH family, plays a crucial role in the catabolic pathways of valine and thymine. Dysregulation of ALDH6A1 expression has been linked to a variety of diseases. Methylmalonate semialdehyde dehydrogenase deficiency (MMSDH deficiency), an autosomal recessive disorder, arises from mutations in the ALDH6A1 gene. Additionally, ALDH6A1 has emerged as a biomarker for several types of severe cancer. Despite its significance, the structural and biochemical mechanisms of ALDH6A1 remain poorly explored.
In a study published in the journal Medicine Plus, researchers identified a structural and biochemical mechanism of ALDH6A1. A structural analysis of ALDH6A1 in its apo form at a resolution of 2.75 Å uncovered a tetrameric architecture with tightly interacting monomers. Their findings also indicated that Alda-1, an agonist of ALDH2, enhanced ALDH6A1 activity as well.
Furthermore, the authors showed that ALDH6A1, compared with ALDH2, exhibited a unique binding model with NAD+. These findings might hold promise for the development of targeted therapies aimed at restoring ALDH6A1 activity, thus providing potential value for individuals affected by related diseases.
"Our work for the first time identifies the structure of the ALDH6A1 apo form, the interaction mechanism between ALDH6A1 and NAD+ by molecular docking, and Alda-1 as an agonist for ALDH6A1," said corresponding author Dr. Xiaodong Luan. "The study for the structural and biochemical mechanism of ALDH6A1 could lay the groundwork for developing targeted therapies to restore ALDH6A1 activity, potentially benefiting individuals affected by related diseases."
More information: Gengchen Su et al, Structural and biochemical basis of methylmalonate semialdehyde dehydrogenase ALDH6A1, Medicine Plus (2024). DOI: 10.1016/j.medp.2024.100008
Provided by Science China Press