This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New study examines ATP synthase at acidic state to reveal how the enzyme functions
A collaborative effort led by Stuti Sharma, Ph.D., Assistant Professor in the Department of Biochemistry & Cell Biology at Stony Brook University, resulted in a promising study toward a better understanding of mitochondrial adenosine triphosphate (ATP) synthase. The work is highlighted in a paper published this month in Nature Structural & Molecular Biology.
Until now, structural studies on the ATP synthase, which is responsible for more than 90% of energy production in living cells, had only been conducted at a basic or neutral potential of hydrogen (pH) level.
Mitochondria often turn acidic in cells affected by diseases such as cancer and cardiac ischemia, as these conditions cause body tissues to become oxygen-deficient or hypoxic. Currently, ATP Synthase is a drug target for various infectious diseases, cardiovascular diseases and cancer. For instance, bedaquiline (Sirturo) is an FDA approved drug that targets bacterial ATP synthase and is prescribed against tuberculosis. So, the potential to broaden ATP synthase-targeted drugs may be viable with additional research.
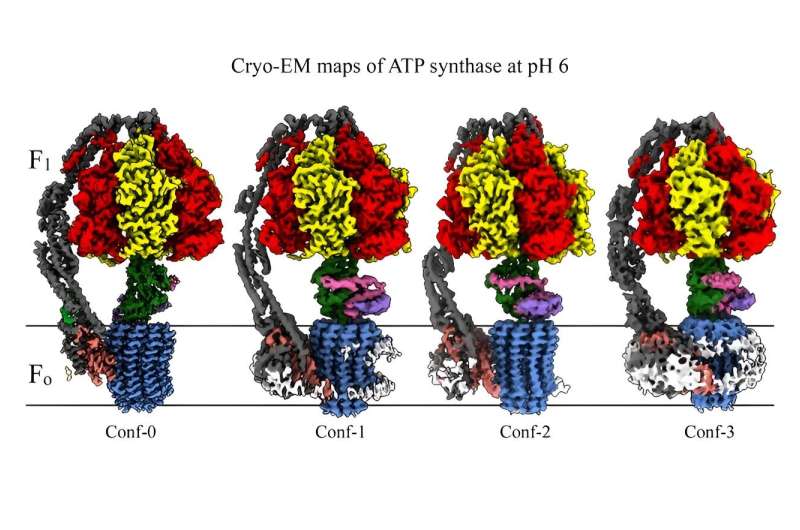
Sharma's study examined the ATP synthase at an acidic state just below neutral on the pH scale. By examining ATP synthase in this way, the researchers uncovered several new insights into how the enzyme functions.
They found four conformations that occur when the ATP synthase is exposed to an acidic environment. Three of these conformations represent distinct stages in the enzyme's reaction cycle, with two unique states that have not been described before. The study illuminates how the ATP synthase operates under hypoxic conditions.
"To understand the mechanism of action of drugs or to design drugs that target molecular machines, it is important to elucidate the basic working mechanism of that molecule," says Sharma. "Our study has improved the understanding of the working mechanism of ATP synthase, especially at low pH."
She hopes this research is an initial step toward the development of new therapies and treatments for the many diseases in which the ATP synthase is implicated.
More information: Stuti Sharma et al, Conformational ensemble of yeast ATP synthase at low pH reveals unique intermediates and plasticity in F1–Fo coupling, Nature Structural & Molecular Biology (2024). DOI: 10.1038/s41594-024-01219-4
Provided by Stony Brook University