This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Advancing towards sustainability: Turning carbon dioxide and water into acetylene
Reaching sustainability is one of humanity's most pressing challenges today—and also one of the hardest. To minimize our impact on the environment and start reverting the damage humanity has already caused, striving to achieve carbon neutrality in as many economic activities as possible is paramount. Unfortunately, the synthesis of many important chemicals still causes high carbon emissions.
Such is the case of acetylene (C2H2), an essential hydrocarbon with a plethora of applications. This highly flammable gas is used for welding, industrial cutting, metal hardening, heat treatments, and other industrial processes. In addition, it is an important precursor in the production of synthetic resins and plastics, including PVC. Since the production of C2H2 requires fossil fuels as feedstock, a more environmentally friendly synthesis route is urgently needed.
Against this backdrop, a research team based on an academia–industry collaboration between Doshisha University and Daikin Industries, Ltd., Japan, has been developing a new and promising strategy to produce C2H2 using carbon dioxide (CO2) and water (H2O) as raw materials.
Their latest study, which included Assistant Professor Yuta Suzuki from Harris Science Research Institute and Professor Takuya Goto from the Department of Science of Environment and Mathematical Modeling of Graduate School of Science and Engineering, both at Doshisha University, and Tomohiro Isogai from Technology and Innovation Center at Daikin Industries Ltd., is published in ACS Sustainable Chemistry & Engineering.
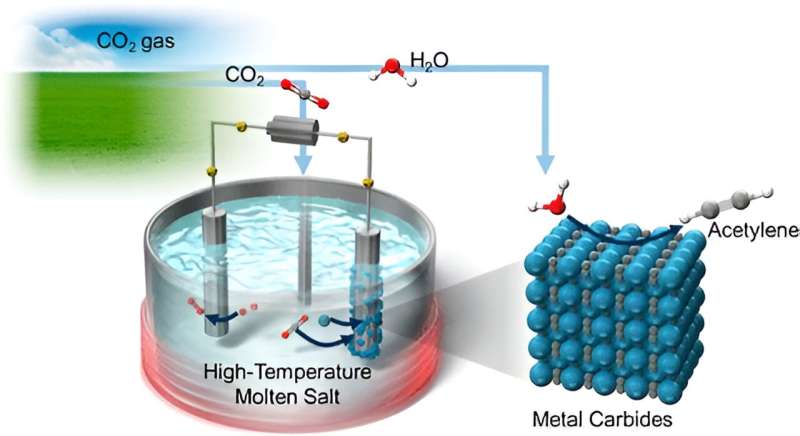
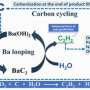
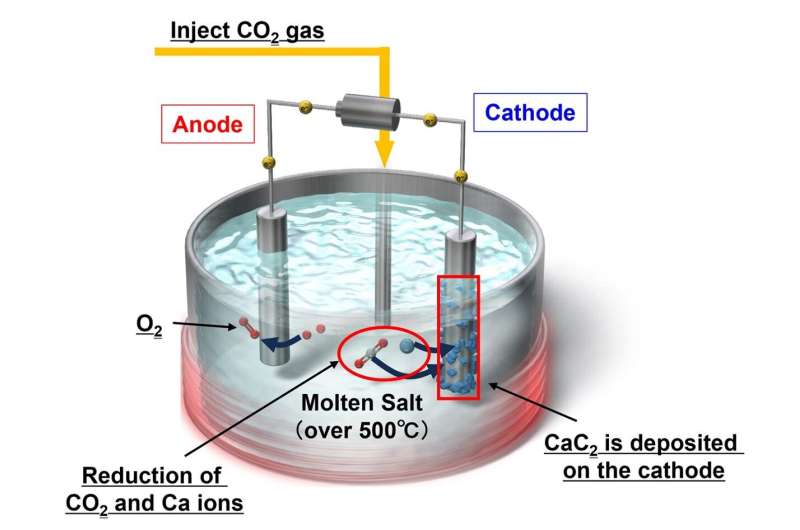
The proposed approach is based on the electrochemical and chemical conversion of CO2 into C2H2 by using high-temperature molten salts, namely chloride melts. One key aspect of the process is that it leverages metal carbides, which are solids composed of carbon atoms and metal atoms, as a pivot point in the conversion.
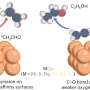
"In our strategy, CO2 is first converted to metallic carbides such as CaC2 and Li2C2, which deposit onto one of the electrodes," explains Dr. Suzuki. "Then, these metal carbides react with H2O, generating C2H2 gas."
To achieve higher energy efficiency out of this method, the team had to test various configurations, including different electrode materials and molten salt compositions. After a series of comprehensive experiments, including cyclic voltammetry, carbon crystallinity analysis, and X-ray diffraction, they determined that a NaCl−KCl−CaCl2−CaO melt saturated with additional CaCl2 in a CO2 atmosphere yielded the best results. This particular melt led to the selective formation of CaC2 around the cathode, which achieved better results than melts including lithium.
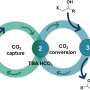
This innovative strategy offers important advantages over conventional synthesis pathways for C2H2. First, the electrodes can be reused after a simple reconditioning treatment since the desired reaction occurs on the deposited metal carbides rather than directly on the electrode surfaces. Another advantage, and perhaps the most notable, is the direct use of CO2 as feedstock to produce an industrially useful and valuable chemical.
"The proposed approach represents a promising technology for realizing a sustainable resource and energy cycle without relying on fossil fuels," says Prof. Goto. "In the future, this same technique could be used as a carbon negative emission technology by extracting carbon dioxide from the air and using it as a raw material, particularly in combination with direct air capture processes."
With any luck, further research on this exciting method will lead to both economically and environmentally viable ways to produce important resins and chemicals from CO2, paving the way to sustainable societies. Ultimately, these efforts would enable us to live in harmony with the environment while maintaining many of the positive aspects of our modern way of life.
More information: Yuta Suzuki et al, New Route of Acetylene Synthesis via Electrochemical Formation of Metal Carbides from CO2 in Chloride Melts, ACS Sustainable Chemistry & Engineering (2024). DOI: 10.1021/acssuschemeng.3c08139
Journal information: ACS Sustainable Chemistry & Engineering
Provided by Doshisha University