This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Controlling the microenvironment to promote wound healing and regeneration
The Korea Research Institute of Standards and Science has unveiled a new principle for controlling the microenvironment of biological tissues to promote wound healing and regeneration. This discovery holds significant promise for the development of wound healing medication as well as research on fibrotic diseases and cancer.
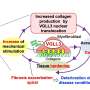
The KRISS Nanobio Measurement Group elucidated the mechanism of fibrosis in wound healing and regeneration through research using skin cells. In addition, they proposed a method of mechanically controlling the microenvironment of biological tissues surrounding wounds to regulate fibrosis at the local level.
Fibrosis is a phenomenon in which the extracellular matrix surrounding cells secretes substances such as collagen, leading to the stiffening of biological tissues. One of the typical examples is the formation of scar tissue in wounds. When this occurs normally, it plays a crucial role in wound healing and regeneration. However, excessive fibrosis can lead to conditions where organs, such as the liver, lungs, and heart, become rigid or can result in autoimmune diseases such as scleroderma.
Fibrosis occurs as fibroblasts differentiate into myofibroblasts. To control fibrosis, therefore, it is essential to understand the conditions within the body that trigger this differentiation.
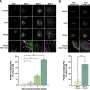
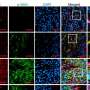
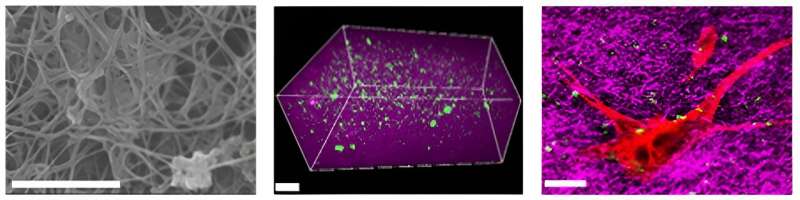
According to the KRISS research team's observation using optical microscopy, fibroblast differentiation is most active when the elastin content of the skin extracellular matrix reaches 20%. The normal level of elastin is 10%, and an increase in this value enhances the elasticity of biological tissue. The results validate the importance of compositional changes in the surrounding microtissues for controlling fibrosis.
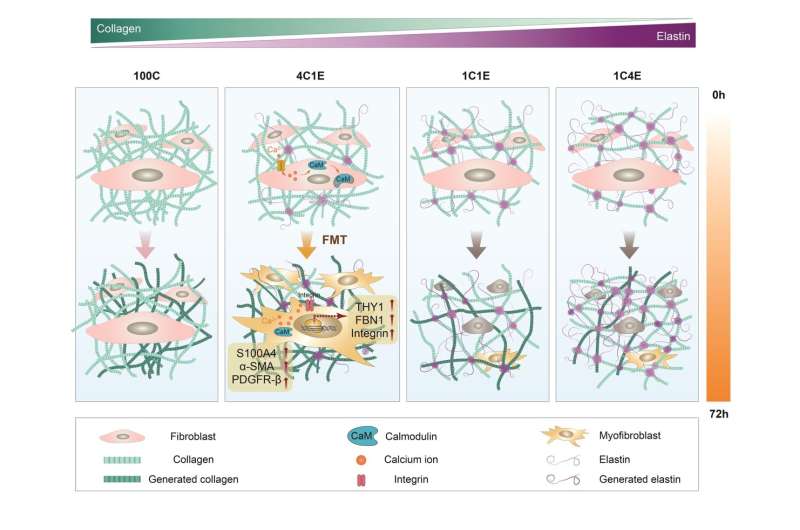
Furthermore, the research team identified proteins involved in regulating the elasticity of biological tissues using high-precision protein analysis and proved that regulating these proteins can promote fibroblast differentiation through experiments.
Conventional research on fibrosis control has employed chemical methods, which is adding growth factors such as EGF to cells to promote fibroblast differentiation. In contrast, the new accomplishment by KRISS involves changing the elasticity of biological tissues in the local area to regulate fibroblast differentiation. This is considered safer than the previous method as it prevents unexpected chain reactions that growth factors might induce within cells.
This achievement was made by the integration of KRISS's unique technologies for nonlinear optical imaging and protein analysis. Nonlinear optical imaging technology enables the observation of collagen within samples without staining, which prevents damage to minute samples during the staining process. Protein analysis technology, which is for the accurate quantification of proteins present in biological samples, provides information on cellular proteins based on elastin content.
This achievement can be applied not only to the development of supplemental medication for wound healing by controlling the cellular microenvironment but also to additional research on treatments for related diseases such as liver fibrosis, lung fibrosis, and heart fibrosis. Since the quantity of elastin is known to influence the proliferation of cancer cells, the findings are also expected to contribute to research on controlling cancer growth.
Dr. Se Hwa Kim, a principal researcher from the KRISS Nanobio Measurement Group, stated, "The convergence of advanced bio-measurement technologies by KRISS led to this achievement. We plan to expand research into various fibrosis mechanisms using organ cells as well as skin cells."
The research is published in the journal Biomaterials Research.
More information: Nhuan T. Do et al, Time-sequential fibroblast-to-myofibroblast transition in elastin-variable 3D hydrogel environments by collagen networks, Biomaterials Research (2023). DOI: 10.1186/s40824-023-00439-x