This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Greenhouse gas repurposed in novel experiments
Cutting-edge University of Auckland research has converted waste carbon dioxide into a potential precursor for chemicals and carbon-free fuel.
Dr. Ziyun Wang's researchers in the School of Chemical Sciences, in collaboration with researchers at Chinese institutions, have demonstrated a method for turning CO2 into formic acid, reported in the journal Nature.
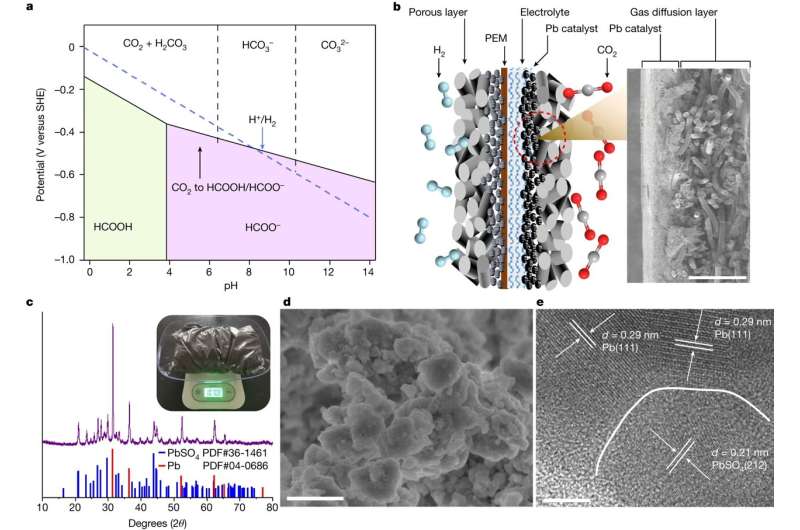
In benchtop experiments, a catalyst made from waste lead-acid batteries enabled a transformation which hadn't been possible using previous catalysts.
Formic acid—the same substance produced by ants ('formica' is the Latin word for ant)—is a colorless and pungent liquid with the potential as a transportation fuel, to store electrical energy, and to enable the petrochemical industry to cut CO2 emissions.
As emissions of carbon dioxide, the primary greenhouse gas, rise each year, scientists are looking into options for the capture and storage of CO2, for repurposing CO2, and for pursuing a carbon-free economy.
Wang's group is one of the world leaders in research into CO2 electrochemical reduction (CO2RR) using acidic rather than alkaline conditions.
"This innovation opens up exciting possibilities for carbon-neutral technologies," he says. "In the future, cars and gas stations could use repurposed carbon dioxide."
In tests, the new method efficiently converted CO2. for more than 5,000 hours, and the researchers' calculations suggest it can be cost-effectively scaled up for industry.
The experiments used a proton exchange membrane electrolyzer. Carbon dioxide flowed into an electrochemical cell and was converted into formic acid, just like charging a battery.
More information: Wensheng Fang et al, Durable CO2 conversion in the proton-exchange membrane system, Nature (2024). DOI: 10.1038/s41586-023-06917-5
Provided by University of Auckland