This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New aging mechanism discovered in nematodes
Even the genes and proteins that have been most closely studied are still far from having given up all their secrets. Like a Swiss Army knife, they have many different, often unknown functions.
A team from the University of Lausanne has focused on MALT1. This protein is known to activate the human immune system when the body is infected. How, then, do we explain that a virtually identical protein is found in C. elegans nematode worms even though it does not play any direct role in the creatures' rudimentary immune defenses?
A very old protein
If we visualize evolution as a tree, the branch of nematodes separated from that of humans more than 600 million years ago—even before the emergence of land animals. Given the huge time gap and distance between the two species, it would be reasonable to expect MALT1 to have either disappeared or mutated to a point where it is barely recognizable. Instead, it is present virtually everywhere in the animal kingdom.
"The fact of its evolutionary conservation got us thinking that the protein might have roles that we hadn't yet considered," explains Margot Thome, a professor at the University of Lausanne and leader of the study published in Autophagy Reports. "We wondered if it might indicate a very old mechanism in evolutionary terms."
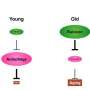
Until now, MALT1 was only known to have neuronal functions in nematodes—including that of activating certain neurons to prevent overexposure to oxygen, which stresses the organism. But this benefit comes at a cost, as the Lausanne-based scientists discovered.
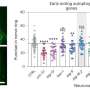
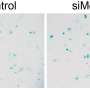
In fact, in the intestine, the protein impedes autophagy, the process by which certain cell components are recycled and an essential part of correct cell functioning. If autophagy is compromised, not only do cells work less well, but the entire organism's life expectancy is reduced.
The researchers found that the nematodes lived longer if MALT1 was deactivated. This effect was even more pronounced under conditions of reduced food intake, where autophagy normally ensures cell survival by recycling unused cell components.
Better understanding of neurodegenerative diseases
The link between autophagy and aging is well documented in humans. As aging progresses, the mitochondria—the cell's "power plants"—are recycled less effectively. This process of decline is associated with muscle weakness and certain common neurodegenerative diseases in the elderly.
Is it possible that MALT1 slows autophagy and shortens lifespan in humans too? For the time being, scientists have yet to study the possible link between the protein and autophagy in humans, Julie Vérièpe, lead author of the study, explains.
"The only established commonality between nematodes and humans is that autophagy declines with age," the researcher tells us. She emphasizes that it is too early to establish a relationship between autophagy and MALT1 in humans, but it is an interesting avenue of exploration from a therapeutic perspective.
As for Thome, she envisages future scientific investigations, initially in worms. For example, it would be important to discover what controls MALT1 activity in C. elegans nematode worms. "In humans, the mechanism is triggered when a pathogen is detected, and the immune system is activated. But we still don't know what happens in worms."
More information: Julie Vérièpe-Salerno et al, MALT-1 shortens lifespan by inhibiting autophagy in the intestine of C. elegans, Autophagy Reports (2023). DOI: 10.1080/27694127.2023.2277584
Provided by Fermi National Accelerator Laboratory