This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
German scientists develop new mutasynthesis approach for derivatization of antibiotics
A new method for the derivatization of antibiotics has been developed by Professor Dr. Yvonne Mast, head of the Department of Bioresources for Bioeconomy and Health Research, and her working group at the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures.
Antibiotics are medicinally-important compounds often produced by microorganisms. Such natural substances often have a chemically complex structure and hence can be difficult or even impossible to chemically synthesize or modify by means of semi-synthesis. However, adapting these substances is often necessary to improve efficacy or, as in the case of antibiotics, to confer resistance-breaking properties.
Mutasynthesis offers an alternative to the chemical modification or "derivatization" of substances. This approach generates mutants of antibiotic-producing microorganisms, wherein the genes for the antibiotic precursor(s) are inactivated, so that the microorganism can no longer produce them.
By "feeding" mutants with modified pre-products (the precursor derivates), these are then incorporated into the antibiotic precursor molecule, thus resulting in the production of new antibiotic derivatives.
Mutasynthesis: An approach to modify antibiotics
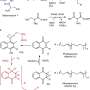
In a study recently published in RSC Chemical Biology, Prof Mast's working group describes a new mutasynthesis approach to derivatization of the antibiotic pristinamycin I. Pristinamycin is a streptogramin antibiotic used as an emergency drug against resistant pathogens.
"We modified pristinamycin I based on the amino acid precursor phenylglycine by mutasynthesis," explains antibiotics researcher Yvonne Mast.
"This was only possible because we had previously identified and functionally characterized the phenylglycine biosynthesis genes, allowing us to generate two new halogenated bioactive pristinamycin I derivatives in our current study."
"The novelty of this study lies in the fact that we coupled a biotransformation process to mutasynthesis, in which the phenylglycine derivative precursor is provided by a genetically modified bacterial strain (E. coli strain). So far, this is the only biotechnological process of its kind, which we have named mutasynthesis 2.0," says Prof Mast.
More information: Oliver Hennrich et al, Biotransformation-coupled mutasynthesis for the generation of novel pristinamycin derivatives by engineering the phenylglycine residue, RSC Chemical Biology (2023). DOI: 10.1039/D3CB00143A
Provided by Leibniz-Institut DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH