This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers experimentally determine the reaction mechanism for catalytic ammonia production

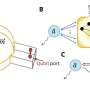
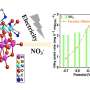
Researchers at Stockholm University have for the first time been able to study the surface of iron and ruthenium catalysts when ammonia is formed from nitrogen and hydrogen. The study, "Operando Probing of the Surface Chemistry During the Haber-Bosch Process," is published in Nature.
A better knowledge of the catalytic process and the possibility of finding even more efficient materials opens the door for a green transition in the currently very CO2-intensive chemical industry.
Ammonia, produced in the Haber-Bosch process, is currently one of the most essential base chemicals for the world to produce fertilizers, with an annual production of 110 million tonnes. The journal Nature proposed in 2001 that the Haber-Bosch process was the most critical scientific invention for humankind during the 20th century, since it has saved around 4 billion people's lives by preventing mass starvation. An estimation of the nitrogen content in our bodies' DNA and proteins shows that half of the atoms can be derived from Haber-Bosch.
"In spite of three Nobel Prizes (1918, 1931, and 2007) for the Haber-Bosch process, it has not been possible to experimentally investigate the catalyst surface with surface-sensitive methods under real ammonia production conditions; experimental techniques with surface sensitivity at high enough pressures and temperatures had not been achievable," says Anders Nilsson, professor of Chemical Physics at Stockholm University.
"Consequently, different hypotheses about the state of the iron catalyst as being metallic or in a nitride, as well as the nature of the intermediate species of importance to the reaction mechanism, could not be unambiguously verified."

"What enabled this study is that we have built a photoelectron spectroscopy instrument in Stockholm that allows studies of catalyst surfaces under high pressures. Thereby, we have been able to observe what happens when the reaction occurs directly," says David Degerman, Postdoc in Chemical Physics at Stockholm University.
"We have opened a new door into understanding ammonia production catalysis with our new instrument where we now can detect reaction intermediates and provide evidence for the reaction mechanism."
"To have our Stockholm instrument at one of the brightest X-ray sources in the world at PETRA III in Hamburg has been crucial to conduct the study," says Patrick Lömker, Researcher at Stockholm University. "We can now imagine the future with even brighter sources when the machine upgrades to PETRA IV."
"We now have the tools to conduct research leading to new catalyst materials for ammonia production that can be used better to fit together with electrolysis-produced hydrogen for the green transition of the chemical industry," says Anders Nilsson.
"It is inspiring to conduct research on a topic that is so linked to a scientific success story that has helped humanity tremendously. I am eager to continue research to find new catalysts that can lessen our dependence on fossil sources. The chemical industry alone accounts for 8% of the world-wide CO2 emissions," says Bernadette Davies, Ph.D. student in Materials Chemistry at Stockholm University.
"The long-term prospect of carrying out ammonia production through an electrocatalytic alternative that is directly driven by solar, or wind electricity is most appealing, and now we have tools to scientifically assist in this development," says Sergey Koroidov, Researcher at Stockholm University.
The study was conducted in collaboration with Deutsches Elektronen-Synchrotron (DESY) in Hamburg and the Montan University in Austria. The study included former employees at the University, Chris Goodwin, Peter Amann, Mikhail Shiplin, Jette Mathiesen and Gabriel Rodrigez.
More information: Anders Nilsson, Operando probing of the surface chemistry during the Haber–Bosch process, Nature (2024). DOI: 10.1038/s41586-023-06844-5. www.nature.com/articles/s41586-023-06844-5
Journal information: Nature
Provided by Stockholm University