This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Is evolution predictable? Bacterial adaptation study seeks to answer this question
A classic question in biology is whether evolution is entirely random or, can it follow predictable patterns. This question was popularized by the famous paleontologist and science communicator Stephen Jay Gould, who in his book "Wonderful Life" wondered what would happen if we could "rewind" the tape of life and let it run again. Would the major phylogenetic groups re-emerge, or would something entirely different happen?
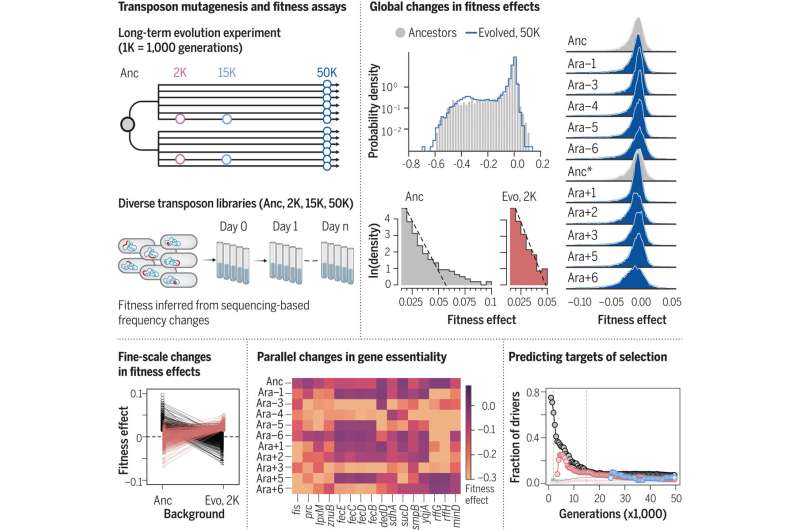
An international study led by Alejandro Couce from the Center for Biotechnology and Plant Genomics (CBGP) at the Polytechnic University of Madrid seeks to answer this question, using bacterial adaptation as an example. The results were published Jan. 26 in the journal Science.
The research sheds light on this classic question by analyzing the evolutionary behavior of experimental populations of bacteria and leveraging the analytical capabilities provided by large-scale, cutting-edge genetic tools. The results reveal that the evolution of bacteria can be predictable in the short term, opening doors to efforts to anticipate the evolution of pathogens and pests, as well as potential biotechnological applications for their control.
The study involved collaboration with the University of Paris (France), the National Institute of Health and Biomedical Research (France), Harvard University (U.S.), the University of Michigan (U.S.), and Imperial College London (United Kingdom).
The main objective of the researchers was to study whether the effect of mutations remains invariant throughout adaptation, or if it shows a significant historical dependence. For example, whether a beneficial mutation in the ancestor becomes harmful in descendants, and vice versa.
"Answering this question has profound implications, the greatest being about the predictability of evolution," explains Alejandro Couce, who heads a lab that studies evolutionary genetics now at UPM.
To deploy this ambitious study, researchers employed recent massive genetic engineering technology that allows the introduction of hundreds of thousands of mutations into bacteria, studying the individual effect of each one individually. "This technology allows exploring the effect, whether good or bad, of all possible mutations along the >4,000 genes of the bacterial genome," adds Couce.
In their work, researchers applied these techniques to the ancestor and different evolutionary stages of the famous Long-Term Evolution Experiment, which has been evolving 12 populations of the same bacteria under constant laboratory conditions for more than 35 years. In total, these populations founded from the same ancestor have experienced >70,000 generations, approximately five times more than Homo sapiens have lived on Earth.
The first significant surprise of this new study is that the overall proportion of lethal, harmful, and neutral mutations remains virtually constant throughout the evolution of these 12 lineages, despite the specific identity of the mutations showing great volatility.
For researchers, a case of particular relevance is lethal mutations: Mutations that, as the name implies, lead to the death of the organism, revealing which genes and systems are essential for life. The results show that many lethal genes in the ancestor cease to be lethal in evolved strains, but a similar fraction of non-lethal mutations in the ancestor becomes lethal later. The result, as Couce explains, is that "the fraction of lethal mutations has enigmatically remained constant during evolution."
This constancy requires rethinking models of the minimum genome concept and has practical implications in both biotechnology (creation of synthetic bacteria) and medicine (search for targets for antibiotics that remain constant in the long term).
The other major result of the study concerns beneficial mutations. These mutations represent the least abundant class of possible effects and are the only ones whose proportion changes throughout adaptation.
"We started with an almost philosophical approach: if we could know all possible beneficial mutations for an organism at a given time, could we predict adaptation?" says the UPM researcher. "It can be seen as a biological version of Laplace's Demon, the thought experiment in which the famous French physicist wondered if for a superhuman intelligence capable of knowing the position and movement of every atom in the universe, it would not be trivial to reconstruct the past and predict the future."
"Our results show that major initial adaptations are predictable, and as evolution progresses, this ability is lost," he explains. "In other words, the demon exists but is terribly shortsighted."
Since major initial adaptations make the difference between survival and extinction, the results obtained in this macro-study provide a "boost" to efforts to predict the evolution of pathogens. This can be applied, for example, to bacteria with multiple antibiotic resistances or new pandemic viruses, or the development of new methods for disease control in agriculture.
Additionally, they demonstrate that these new massive genetic engineering techniques could be used to develop microbes adapted to different demands or applications in record time. For example, it would be possible to design bacteria that protect plants from other pathogens or bacteria that produce or degrade compounds of interest more effectively.
"Our work opens the door to dreaming that a theory of evolution capable of making concrete predictions is possible, even if only at a statistical level, as is the case with climate science," concludes Couce.
More information: Alejandro Couce et al, Changing fitness effects of mutations through long-term bacterial evolution, Science (2024). DOI: 10.1126/science.add1417
Journal information: Science
Provided by Universidad Politécnica de Madrid