This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
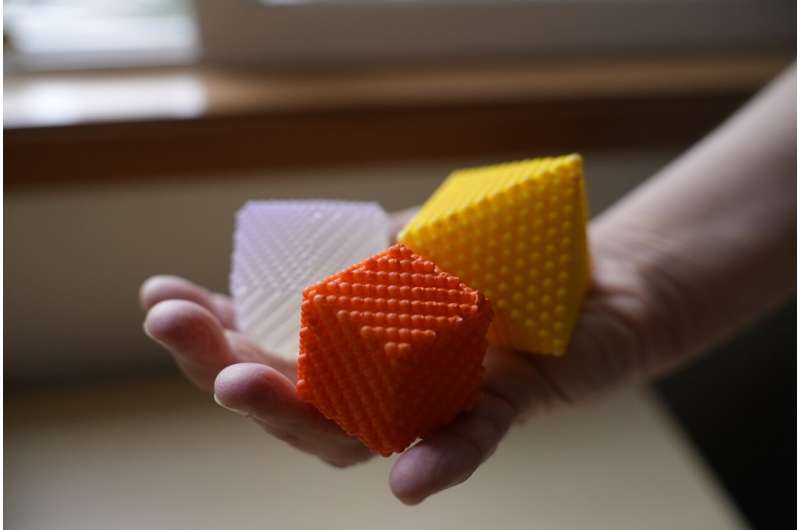
3D models for placing nanoparticles in the palm of your hand
Nanoparticles are super tiny―as small as one nanometer, or one billionth of a meter―and are of keen interest to materials scientists for their unique physical and chemical properties. They cannot be detected by the naked eye and require a highly specialized electron microscope to be seen.
In fact, advancements in imaging technologies through the 1990s and early 2000s are what made the field of nanoscience possible, says Anne Bentley, a faculty member in the Department of Chemistry at Lewis & Clark College in Portland, Oregon.
"I think a lot of chemistry is outside the realm of what people can hold in their hands," she says. "You can obtain evidence about what's going on, but you're still investigating something that's at too small a scale for your eyes to see. Anything you can do to scale it up is helpful."
So Bentley did just that, creating 3D models of the simplest geometric shapes that nanoparticles form. She has made the instructions for creating these models, either with paper or 3D-printing material, available as part of an article she co-authored, published in the Journal of Chemical Education, called "A Primer on Lattice Planes, Crystal Facets, and Nanoparticle Shape Control."
A primer for materials chemistry students
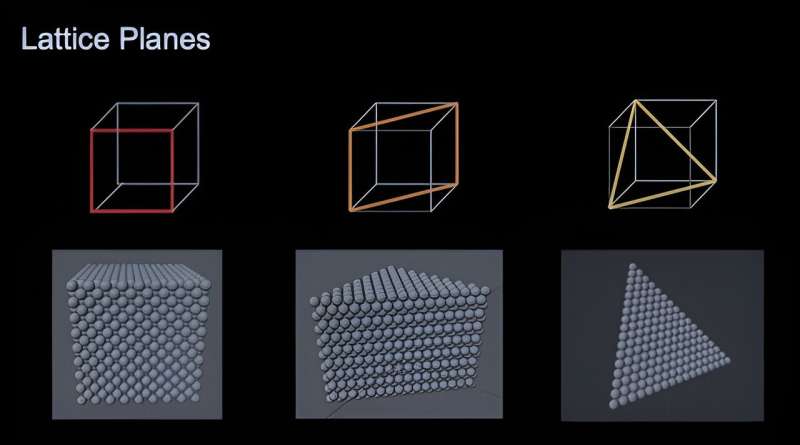
Nanoparticles come in different geometric shapes and are crystalline or composed of atoms arranged in a pattern that repeats in three dimensions. The shapes display flat surfaces, called planes or facets, similar to the cuts in a gemstone. The arrangement of atoms on these crystal surfaces influences the material's special properties, says Bentley.
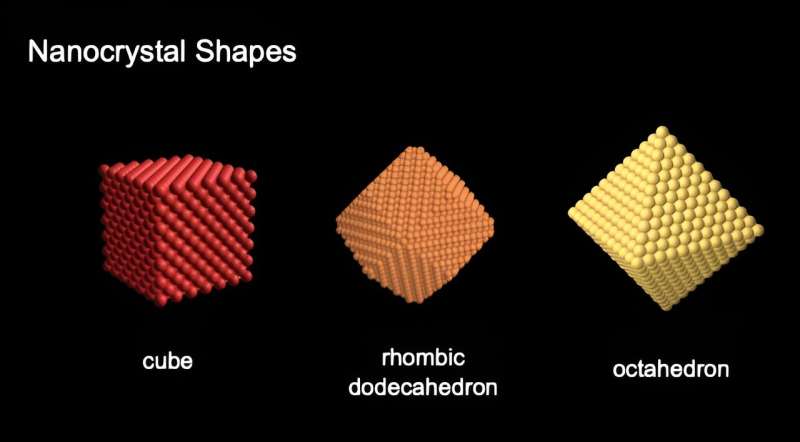
"The shapes are derived from this packing of the atoms," she says. "The motivation to make different shapes really comes down to the arrangement of the atoms when the material is sliced in different ways on different crystal planes."
In the paper, Bentley focuses on low-index shapes, which she describes as the three simplest ways to slice the structure.
"There are lots more complex ways to slice it, but these are the three fundamental ways to do it, by making them either six, eight, or twelve sides―cubes, octahedra, or rhombic dodecahedra. It was a natural choice to focus on those three for the article."
Transforming a 'jumble of numbers' into shapes
"Nanoscience is a topic that both falls between chemistry and physics in the curriculum, but also between undergraduate- and graduate-level research," says Bentley.
"It's important that beginning materials chemists have a fundamental understanding of crystal planes, facets, and directions of growth. They also need to understand the three-digit notation system used to index these attributes, known as the Miller indices. Otherwise, this system can look like a mysterious jumble of numbers."
She felt it was important to provide a foundation of knowledge in an accessible format that could aid educators in introducing this important and growing field. While more complex structures than the 3D-printed models can be created digitally via computer simulation programs, Bentley believes that there are advantages to being able to hold the models in your hands.
"I like things I can look at and think about," she says, adding that 3D models are particularly useful for generating an understanding of this key nanoscience topic.
Growing gold particles to convert carbon dioxide
In Bentley's lab, she and students work on manipulating gold atoms in vials of liquid to control the nanoparticle shapes.
"You need to just make the right conditions at the right temperatures, a whole environment that is conducive to growing a particular shape," she says.
Bentley studies gold nanoparticles, which are notable for their catalytic properties or ability to accelerate chemical reactions. The way the material is sliced exposes different patterns of atoms, she explains. Previous research has identified that one particular gold nanoparticle shape, the 12-sided rhombic dodecahedra, is more effective for converting carbon dioxide into fuel materials.
"It's like recycling," says Bentley. "Not only does this nanoparticle shape enable researchers to remove carbon dioxide from the atmosphere, but it allows them to turn it back into some kind of fuel that can be used. So if we can grow particles that have this facet on them only, that's a real advantage."
More information: Anne K. Bentley et al, A Primer on Lattice Planes, Crystal Facets, and Nanoparticle Shape Control, Journal of Chemical Education (2023). DOI: 10.1021/acs.jchemed.3c00371
Journal information: Journal of Chemical Education
Provided by Lewis & Clark College