This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Study shows what kind of salt we use on the roads in winter can make a difference for plants

Increased salinity in soils is a global problem caused, for example, by ions leaching into soils from ice-melting salts used on roads in winter or from seawater seepage in coastal areas.
Salinization of farmland affects more than one and a half billion people worldwide. Researchers at Eötvös Loránd University (ELTE) investigated how high salt concentrations affect the development of wheat seedlings, which germinate deep in the soil, and what can be done to reduce the damage caused by salinization.
Increased salinity in soils is a global problem in agriculture, but also affects local crop production in gardens and in farmlands close to roads where large amounts of salts are used in winter as ice-melting agents, and in coastal areas where seawater seepage may occur. In nature, the leaves of seedlings growing in the dark deep in saline soils can be directly exposed to different ions in the soil, which can cause plant stress and ultimately plant death.
Researchers from Eötvös Loránd University have shown that greening, that is, the biosynthesis of the green pigment, chlorophyll, and the development of photosynthetically active plastids, called chloroplasts, are inhibited in wheat leaves grown in the dark and then directly exposed to high salt concentrations. This can eventually lead to the death of the seedlings.
Katalin Solymosi and her research group have used a very simple and reproducible method to easily identify which salts and in what concentrations have the most harmful effects on the greening of plants germinating in soil. Sodium (Na+) was found to have the most negative effect, while potassium (K+) salts (e.g., in the form of wood ash, traditionally often used for such purposes) and calcium chloride (CaCl2) can be considered as more environmentally friendly ice-melting compounds.
A global problem
Increased soil salinity affects an estimated 833 million hectares of land worldwide. Unfortunately, due to inappropriate agricultural practices, this area is constantly increasing. The concentration of salts and the available water is constantly changing in the soil depending on several factors, including the quality and quantity of rainfall, external temperature, and evaporation.
"Many researchers have been investigating for a long time how high soil salinity inhibits the germination of most economically important crops and reduces their yields. However, no studies are available about how saline soils affect the leaves of plant seedling developing from seeds sown deep into the soil according to agricultural planting protocols of many crops."
"This is even more surprising as salinization of croplands affects about one and a half billion people worldwide," explains Katalin Solymosi, assistant professor at Eötvös Loránd University and lead researcher of the study.
The invisible death of wheat
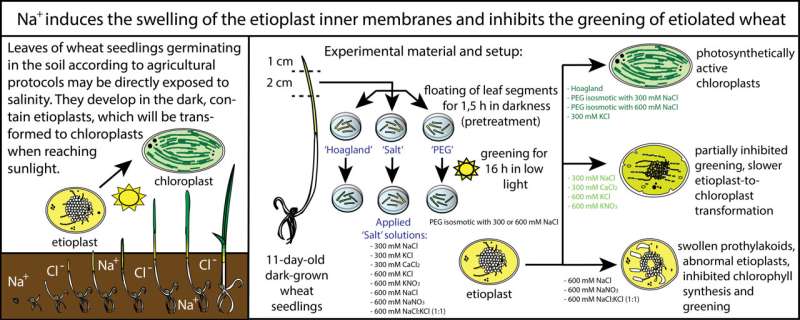
Depending on the soil type and the used wheat cultivar, wheat is usually sown 5–10 cm deep into the soil. Thus, the germinating wheat seedlings first develop in the soil in complete darkness. Due to the lack of sunlight, they do not produce the green pigment chlorophyll, which is characteristic of plants, and are therefore yellowish in color.
Without chlorophyll, the photosynthetic apparatus cannot develop, and a specific plastid type, the so-called etioplast develops in the leaves of these seedlings. When the leaves of developing plants reach the soil surface and are gradually exposed to light, chlorophyll is synthesized in them, the plants turn green and the etioplasts in them are transformed into green plastids, called chloroplasts. Chloroplasts are photosynthetically active and help the plant to produce sugars and thus energy for survival.
Without the switch to self-sustaining photosynthesis, seedlings will eventually use up the nutrients stored in their seeds and die.
"In this context, it is particularly surprising that no study has so far investigated in detail how high salt concentrations affect the greening of wheat seedlings, even though it is obvious that the leaves of plants germinating in soil are directly exposed to soil salinity," says Adél Sóti, Ph.D. student at Eötvös Loránd University, first author of the study published in the journal Planta.
"We found a simple method to investigate how salt affects the greening of plants. After 11 days of germination in complete darkness, for example in a closed box, leaf pieces of seedlings were first exposed to different concentrations of salt for 1.5 hours as a pretreatment and then illuminated in relatively low light to green them. Greening, that is the appearance or absence of green color after 16 hours of illumination, is readily visible to the eye and indicates the extent to which the saline solution was detrimental to the process."
Salt stress and water shortage
In fact, it is surprising that even half the salt concentration of seawater (i.e., about 300 mM NaCl) does not completely inhibit wheat greening but slows down the transformation of etioplasts into chloroplasts. However, at relatively high salt concentrations (e.g., 600 mM NaCl), equal to the salinity of seawater, greening is completely inhibited and specific structural changes, such as the swelling of the inner membranes of the etioplasts, are observed.

High concentrations of saline solutions have negative effects on plants through at least two main mechanisms: Because they are rich in dissolved ions, they can hinder water uptake and thus cause so-called osmotic stress, and the various salt ions can have a direct toxic effect on cells after uptake and thus interference with their metabolism.
Within the framework of the ERASMUS+ staff mobility program, Hungarian researchers, in collaboration with Beata Mysliwa-Kurdziel from the Jagellonian University in Krakow, conducted systematic studies to test whether the osmotic stress caused by the applied saline solutions significantly contributed to the observed inhibition of greening. For these studies, a non-ionic compound, polyethylene glycol, was used at the same osmolarity as the saline solutions to mimic the osmotic stress caused by the different saline solutions.
Less sodium, higher yield
"With electron microscopic investigations we showed that the large and abnormal swelling of the water-containing inner space (called lumen) of the etioplast inner membranes did not occur during the application of only osmotic stress and was only observed in samples treated with high Na+ concentrations."
"Our comparative analyses showed that high concentrations (600 mM) of KCl, KNO3 or 300 mM CaCl2 also slowed down the greening, but did not induce such marked ultrastructural changes," says Katalin Solymosi.
These results may reinforce the collective wisdom of people to use, for example, wood ash enriched in potassium salts (K+) instead of sodium salts for de-icing snowy roads in winter.
Similarly, CaCl2 is considered a more environment friendly ice-melting and anti-skid agent. At high concentrations, however, neither salts have a positive effect on the greening of seedlings, which is something to bear in mind during winter snowfalls if you want to have a beautiful garden with lots of green plants next spring.
The paper is published in the journal Planta.
More information: Adél Sóti et al, Ionic, not the osmotic component, is responsible for the salinity-induced inhibition of greening in etiolated wheat (Triticum aestivum L. cv. Mv Béres) leaves: a comparative study, Planta (2023). DOI: 10.1007/s00425-023-04255-4
Provided by Eötvös Loránd University