This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Polyoxometalates and ionic liquid enhance solid-state lithium-ion electrolyte performance
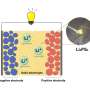
Solid-state lithium-ion batteries depend on the movement of ions (charged atoms) in the solid, rather than liquid, state to either charge or discharge the battery. These solid-state electrolytes are safer, more cost efficient and capable of higher energy densities than batteries that rely on liquid electrolyte solutions, but suffer from low ionic conductivity, or movement of ions, and poor thermal stability.
A new composite solid electrolyte (CSE) membrane has been synthesized by researchers from Northeast Normal University using lithium salts and an ionic liquid to improve the dissociation, and therefore conductivity, of charged lithium atoms in a solid-state electrolyte battery. The team published their results in the journal Polyoxometalates on September 28, 2023.
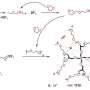
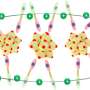
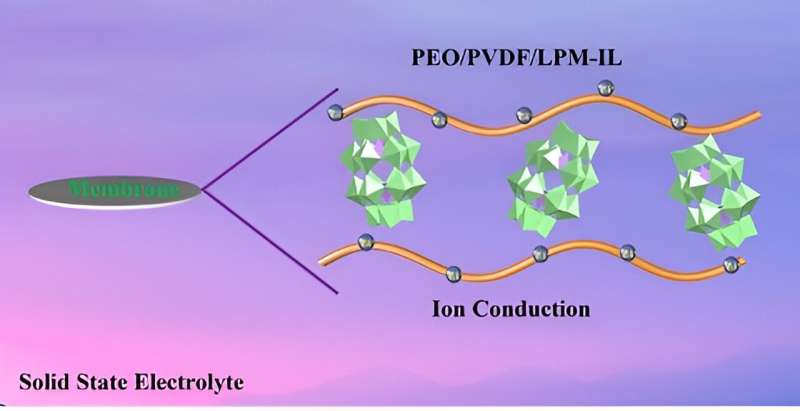
Polyoxometalates (POMs) are clusters of metal and oxygen atoms with properties that are determined by the well-defined structure of the POM atom cluster. The researchers recently introduced a POM-based lithium salt, Li6P2Mo18O62 (LPM) into a solid-polymer electrolyte (SPE) made up of the polymer polyethylene oxide (PEO), an inexpensive and stable chain of many ethylene oxide subunits.
PEO suffers from low ionic conductivity, and the addition of LPM salt alters the properties of the polymer and enhances ion movement. The research team also incorporated an ionic liquid (IL) to free lithium ions from LPM, further improving the conductivity of the composite electrolyte material.
"Solid-state electrolytes (SSEs) are considered… the most promising candidates for next-generation energy storage devices due to their excellent thermal and electrochemical stability. Although SPEs have excellent flexibility and viscosity, they are severely limited due to their low ionic conductivity, poor mechanical strength and low thermal stability at room temperature.
"In contrast, inorganic solid electrolytes (ISEs) like LPM... usually have high ionic conductivity. By incorporating ISEs like LPM into SPEs to form composite polymer electrolytes, we leverage their respective properties… to achieve optimized mechanical properties and improve their ionic conductivity," said Hong-Ying Zang, senior author of the paper and professor in the Key Laboratory of Polyoxometalate and Reticular Material Chemistry at Northeast Normal University in Changchun, China.
"Currently, inorganic electrolyte fillers include nanoparticles… and ionic-conductive inorganics. As a class of metal-oxygen clusters, the application of polyoxometalates in solid-state batteries is hampered by the difficulty of moving lithium ions. In this paper, we promote the dissociation of lithium ions from polyoxometalates with ILs… to impart LPM and IL composites (LPM-IL) with good electrical conductivity," said Zang.
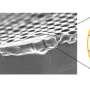
The team characterized the ion conductivity and mobility of the composite membrane by measuring the AC impedance, or the resistance of current flow in a circuit. The team found that electrolyte membranes containing the optimal concentration of LPM and IL demonstrated three times higher conductivity than membranes prepared without IL.
In a similar manner, the team determined that the conductivity of composite membranes generated with polyvinylidene fluoride (PVDF), a non-reactive thermoplastic filler material, in conjunction with PEO increased conductivity ten times compared to LPM-IL membranes synthesized without PVDF. The composite membrane also demonstrated good stability over 12 hours at a temperature of 80℃.
"The results of these experiments demonstrate that polyoxometalates can be used as inorganic solid electrolytes," said Zang. IL effectively increased the dissociation of lithium ions from LPM and improved the ionic conductivity of the composite solid electrolyte membrane. The incorporation of PVDF also created a PEO-PVDF conductive network in the membrane that further promoted lithium ion movement, enhancing conductivity.
The research team believes their unique, PEO-based composite membrane containing PVDF, POM-based lithium salt and IL provides a practical means of increasing ionic conductivity in solid-state electrolytes for use in lithium-ion batteries. "Our next step is to improve the performance of polyoxometalates to create better solid-state lithium-ion batteries," said Zang.
More information: Qianqian Liu et al, Ionic liquid-mediated PEO-based solid-state electrolyte membrane modified with Dawson-type polyoxometalates, Polyoxometalates (2023). DOI: 10.26599/POM.2023.9140036
Provided by Tsinghua University Press