This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Cell-friendly bioprinting at high fidelity enhances its medical applicability
What if organ damage could be repaired by simply growing a new organ in the lab? Improving researchers' ability to print live cells on demand into geometrically well-defined, soft complex 3D architectures is essential to such work, as well as for animal-free toxicological testing.
In a study recently published in ACS Biomaterials Science and Engineering, researchers from Osaka University have overcome prior limitations that have hindered cell growth and the geometrical fidelity of bioprinted architectures. This work might help bring 3D-printed cell constructs closer to mimicking biological tissue and organs.
Ever since bioprinting was first reported in 1988 by using a standard inkjet printer, researchers have explored the potential of this layer-by-layer tissue assembly procedure to regrow damaged body parts and test medical hypotheses. Bioprinting is to eject a cell-containing "ink" from a printing nozzle to form 3D structures. It is usually easier to print hard rather than soft structures. However, soft structures are preferable in terms of cell growth in the printed structures.
When printing soft structures, doing so in a printing support is effective; however, solidification of ink in the support filled in a vessel can result in its contamination with unwanted substances from the support. Ink solidification into a soft matrix using a printing support without contamination, while retaining cell viability, was the goal of this work.
"In our approach, a 3D printer alternately dispenses the cell-containing ink and a printing support," explains Takashi Kotani, lead author of the study. "The interesting point is that the support also plays a role in facilitating the solidification of the ink. All that's necessary for ink solidification is in the support, and after removing the support, the geometry of the soft printed cell structures remains intact."
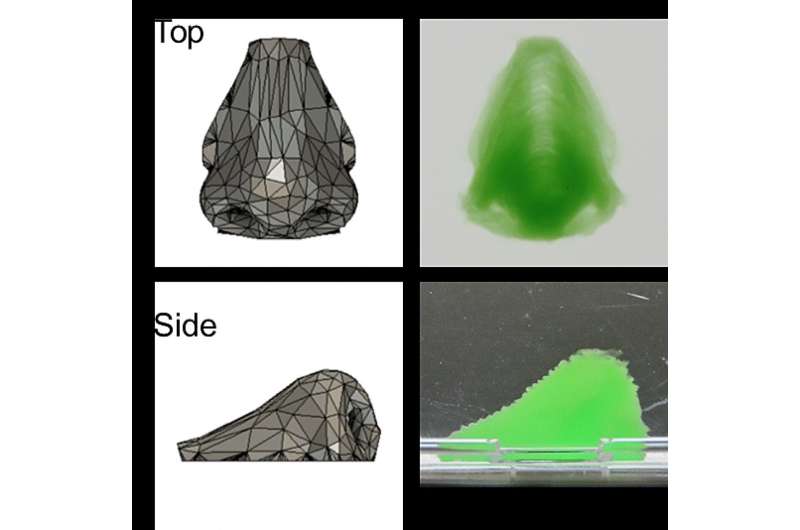
Hydrogen peroxide from the support enabled an enzyme in the ink to initiate gelation of the ink, resulting in a gel-enclosed cell assembly within a few seconds. This rapid gelation prevented contamination of the assembly during formation. After removing the support, straightforward 3D constructs such as inverted trapezium geometries as well as human nose shapes—including bridges, holes, and overhangs—were readily obtained.
"We largely retain mouse fibroblast cell geometry and growth, and the cells remain viable for at least two weeks," says Shinji Sakai, senior author. "These cells also adhere to and proliferate on our constructs, which highlights our work's potential in tissue engineering."
This new technique is an important step forward to engineering human cell assemblies and tissues. Further work might involve further optimizing the ink and support, as well as incorporating blood vessels into the artificial tissue to improve its resemblance to physiological architectures. Regenerative medicine, pharmaceutical toxicology, and other fields will all benefit from this work and further improvements in the precise fidelity of bioprinting.
More information: Takashi Kotani et al, Horseradish Peroxidase-Mediated Bioprinting via Bioink Gelation by Alternately Extruded Support Material, ACS Biomaterials Science & Engineering (2023). DOI: 10.1021/acsbiomaterials.3c00996
Provided by Osaka University