This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers reversely trap isolated atoms in high oxidation to accelerate oxygen evolution reaction kinetics
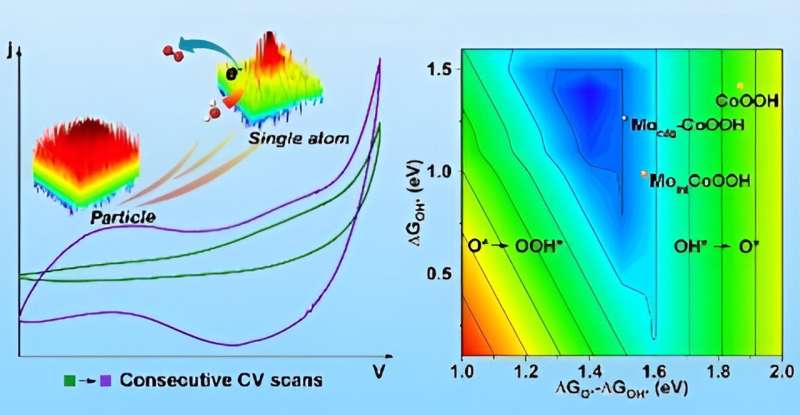
Single-atom catalysts (SACs), representing the ultimate high atom utilization efficiency and specific activity, have arguably become the most active new frontier in heterogeneous catalysis. The technology of atom trapping based on support effects is a viable method for synthesizing SACs.
Because trapping isolated metal atoms is highly desired by researchers, the structural dynamics of materials have been used during catalytic conditions, especially at the dynamical generation stage of active moiety, where the reconstructed atomic-precision catalyst surface usually possesses thermodynamically stable stoichiometry.
In a study published in Angewandte Chemie International Edition, the research group led by Prof. Zhang Jian from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences, in collaboration with Prof. Zhang Huabin from King Abdullah University of Science and Technology, Saudi Arabia, reversely trapped isolated atoms in high oxidation to accelerate the oxygen evolution reaction (OER) kinetics.
The researchers first studied the atomization effects under in-situ OER conditions and elucidated how the incorporation of high-value metal modulators promotes the OER activity of CoOOH. The developed Mo-CoOOH demonstrated enhanced OER activity, kinetics and stability.
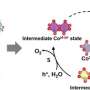
The team then revealed the origin and kinetics of the ultra-fast structural reconstruction during the extraction of isolated Mo atoms from a-MoOx@CoSe2 pre-catalysts by using multi-model operando characterizations. The ultra-fast reconstruction process enabled the Mo atoms to trap in the CoOOH lattice, forming higher covalency Co-O-Mo bonds.
Electrochemical analysis and theoretical calculations verified that the single Mo-atom decoration shifts the oxidation cycle of Co sites toward a more energetically favored rate-limiting process.
In addition, the researchers found that the Mo-CoOOH only needs an extremely low overpotential of 297 mV to achieve a current density of 100 mA cm-2 in alkaline media, exhibiting top-level catalytic activity among all reported electrocatalysts.
This study provides a fundamental understanding of the dynamic reaction pathways of the pre-catalyst and offers more possibilities of scalable single-atom extraction strategies for designing highly active electrocatalysts.
More information: Yang Li et al, Reversely Trapping Isolated Atoms in High Oxidation for Accelerating the Oxygen Evolution Reaction Kinetics, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202309341
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences