September 25, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Targeted nanotherapy: Fluorescence-guided photoimmunotherapy to manage peritoneal carcinomatosis
Fluorescence-guided intervention strategies can improve standard therapies to detect and treat microscopic tumors to thereby prevent lethal recurrence. Cancer biologists have made tremendous progress in photoimmunotherapy and nanotechnology to treat metastasis, although the effects of such techniques are limited by heterogeneous effects.
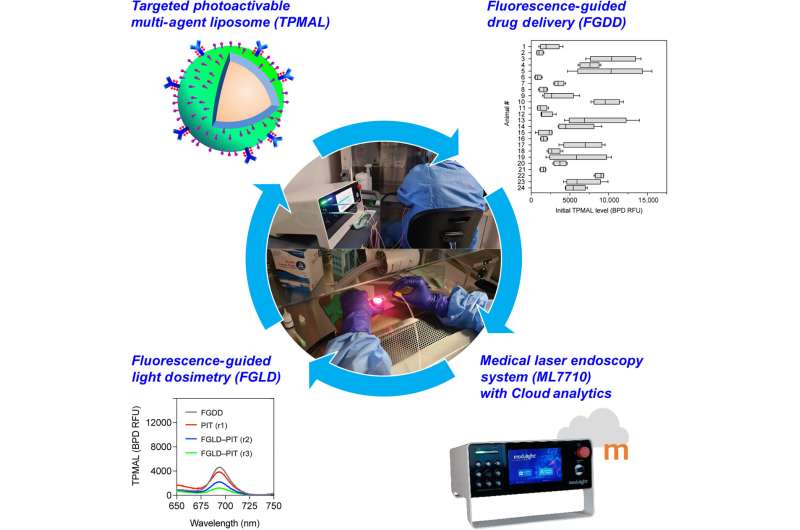
In a new report published in Science Advances, Barry J. Liang, and a team of researchers in bioengineering, cell biology and photomedicine at the University of Maryland, Baltimore, Harvard Medical School U.S. and the Modulight Corporation, Finland, integrated three technical advances for fluorescence-guided intervention in targeted photo-activatable multiagent liposome laser endoscopy for improved photoimmunotherapy.
The photoactivatable multiagent liposome contained a nanoliposome labeled with fluorophores to track and photosensitize immunoconjugates for photoimmunotherapy. The researchers conducted fluorescence-guided drug delivery during the experiments and fluorescence-guided light dosimetry to investigate peritoneal carcinomatosis in mouse models.
Fluorescence-guided drug delivery methods revealed that the targeted photoactivatable multiagent liposome enhanced drug delivery to metastases increased by 14-fold. The team combined the interventional methods to vary the treatment response for tumor control without side-effects.
Photodynamic therapy for ovarian cancer
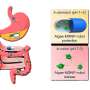
Peritoneal metastasis or incomplete resection and drug resistance can make advanced ovarian cancer virtually incurable with the existing approaches in surgery and chemotherapy. While tumor recurrence is nearly universal, the five-year survival rate of 30% has not significantly changed in the past three decades.
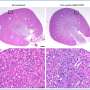
At diagnosis, up to 70% of these patients are in advanced stages. The primary mechanism of serous carcinoma metastasis involves the high-grade deposition of numerous cancer nodules throughout the abdominal cavity. Women with advanced ovarian cancer who undergo surgery and chemotherapy have achieved complete remission, although the patients relapse due to residual sub-mm lesions.
While such aggregates are difficult to detect, they can develop resistance to standard treatments, therefore radical approaches that combine targeted therapy, imaging and monitoring should address drug-resistant micro-metastases.
Using targeted photoactivatable multiagent liposomes (TPMAL)
Although intraoperative photodynamic therapy for peritoneal carcinomatosis using non-targeted photosensitizers and a fixed light dose combination is safe for clinical use, the technology has not yet achieved complete responses or long-term tumor control due to tumor heterogeneity and a lack of specificity during the uptake of photosensitizer.
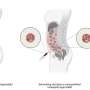
In this work, Liang and colleagues used targeted photoactivatable multiagent liposomes (TPMAL) for photochemotherapy engineered with molecular targeting and fluorescence-tracking features. The scientists integrated a laser endoscopy system to advance a two-pronged approach to ensure TPMAL-assisted photodynamic therapy for safe, and customized drug delivery. The outcomes highlight the efficiency and safety of photoimmunotherapy to reduce the metastatic burden in vivo.
Activating photoimmunotherapy in the lab
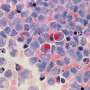
To activate photoimmunotherapy, the scientists covalently attached five benzoporphyrin derivative photosensitizer molecules to each anti-epidermal growth factor receptor monoclonal antibody through carbodiimide chemistry. Using click chemistry, they covalently linked photoimmunotherapy to the nanoliposomes to synthesize targeted photoactivatable multilayer liposomes.
The resulting photosensitizer immunoconjugates-nanoliposomes-carboxyfluorescein compounds (abbreviated PIC-Nal-CF) showed an average size in the nano-scale range with a storage capacity of up to 18 weeks. By conjugating the compound, Liang and colleagues did not alter the absorption of the emission spectra of benzoporphyrin derivatives and carboxyfluorescein. They noted a stronger fluorescent signal at 700 nm from the conjugates and noted the activity of the material to be more than five times greater than the free elements.
In vivo experiments in mice
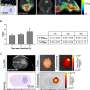
Liang and colleagues used intralipid; a light scattering agent in the clinic for intraperitoneal photodynamic therapy of peritoneal carcinomatosis. They examined intralipid alters and fluorescence emission spectra of the photoimmunotherapy conjugates (PIC-Nal-CF).
Using 0.03% ink as a light absorber, they studied the turbid environment of peritoneal carcinomatosis, where the serosanguinous fluid of the tumor reduced the fluorescence signal from the carboxyfluorescein to benzoporphyrin, while intralipid aided the fluorescence monitoring of the compound.
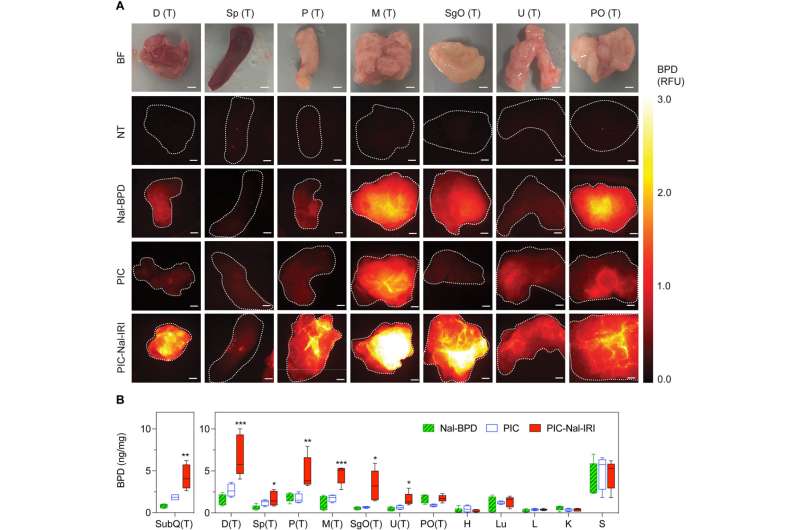
The team studied the biodistribution of photosensitizer immunoconjugate-nanoliposome-carboxyfluorescein compounds in a mouse model of disease and detected the compound in the peritoneal cavity of mice to highlight enhanced tumor selectivity and retention capacity.
Improving the efficiency of photosensitizer immunoconjugates to peritoneal metastasis
The team assessed the biodistribution of the theranostic compound by tracking fluorescence signals to indicate their capacity to "lock-in" and "co-deliver" by designing drug ratios to target sites. When compared to the normal tissues, they observed an increased accumulation of the compound in metastatic tumors and noted high levels of benzoporphyrin accumulation in tumor tissues after injecting the compound of interest.
Photoimmunotherapy is typically limited by a poor uptake of photosensitizer by tumor cells. The team studied whether the photosensitizer and nanoliposomes with irinotecan (PIC-Nal-IRI) could improve the delivery of benzoporphyrin immunoconjugates alone.
After 24 hours of intraperitoneal injection, the animals treated with the conjugates showed the highest accumulation of benzoporphyrin fluorescence signal in the tumor regions with low signals in all healthy tissues. The drug conjugates showed the highest tumor to normal tissue ratios. The team also studied intraoperative fluorescence-guided drug delivery in different animal models.
Outlook
In this way, Liang and colleagues examined the use of photodynamic therapy as a promising treatment strategy for peritoneal carcinomatosis. Ovarian cancer is a lethal gynecologic malignancy in the U.S., with a five-year survival rate of stage 1 disease at 92%, although with a 75% diagnosis of patients at advanced stages and limited treatment options to impact the overall survival rate.
Photodynamic therapy offers a promising treatment strategy for peritoneal carcinomatosis. Photoimmunotherapy is a targeted version of the process introduced in 1983 to enhance tumor selectivity in complex sites such as the peritoneal cavity.
The method can produce cytotoxic reactive oxygen species to kill cancer cells and activate local and systemic antitumor immune responses under appropriate doses of light and photosensitizer.
The cancer-targeted photoactivated multiagent liposomes developed in this work can be co-delivered with photosensitive immunoconjugates and carboxyfluorescein dyes or irinotecan chemotherapy to explore the dynamics of an ideal cancer treatment strategy for drug delivery and treatment by providing insights to the underlying mechanisms of antitumor immune responses to photoimmunotherapy compounds.
More information: Barry J. Liang et al, Fluorescence-guided photoimmunotherapy using targeted nanotechnology and ML7710 to manage peritoneal carcinomatosis, Science Advances (2023). DOI: 10.1126/sciadv.adi3441
Willemien J. van Driel et al, Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer, New England Journal of Medicine (2018). DOI: 10.1056/NEJMoa1708618
Journal information: New England Journal of Medicine , Science Advances
© 2023 Science X Network