This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Modular flow cells for sustainable chemistry

Electrochemical processes like water electrolysis will become increasingly important in the future in light of climate change and the resultant need for an energy and raw materials transition. The Fraunhofer Institute for Microengineering and Microsystems IMM is collaborating with hte GmbH to develop modular electrochemical cells. These flow cells are used in screening tasks, thereby helping to optimize electrochemical production processes, such as water electrolysis.
In the context of the energy and raw materials transition, electrochemical processes pose an advantage, as they run on electricity rather than chemical reagents. They can also actively respond to fluctuations in the power supply by connecting electrochemical processes when there is an excess of electricity and switching them off when electricity is lacking.
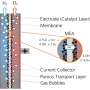
An important use case is water electrolysis. This generates hydrogen—a flexible and easy-to-transport source of energy that plays a central role in the energy transition. For this reason, Fraunhofer IMM is developing and improving electrochemical microreactors—also known as "flow cells"—for use in green synthesis processes like electrosynthesis and as a means of studying water electrolysis.
These microreactors enable the development and screening of the syntheses on a laboratory scale, while simultaneously allowing the synthesis to be transferred to pilot scale. Alongside Heidelberg-based hte GmbH, Fraunhofer IMM has developed a modular and flexible concept for an electrochemical flow cell that they have put to use for high-throughput screening tasks in electrocatalysis.
"Chemical processes can be precisely controlled with the help of microreactors. This also applies for our electrochemical cells, which can be used to optimize electrochemical production processes, for example, when it comes to sustainable chemical production or water electrolysis. Screening platforms that use our design allow for a number of catalysts and processing conditions to be tested in a very short time.
"Modifications need to be made to screening platforms for electrocatalysis. Aside from the electrocatalyst itself, process conditions such as pressure, temperature, cell voltage, flow rates and electrolyte composition also need to be investigated. This is where our flow-through reactors come into play as part of the screening system," explains Dr. Patrick Löb, head of the Flow Chemistry group at Fraunhofer IMM in Mainz.

A plate stack-based reactor concept
The basic reactor concept developed by Fraunhofer IMM for electrochemical applications uses a plate stack design. A single electrochemical cell is made up by a set of electrode plates and other components. The stack may contain either one cell for individual operation, or a set of cells that can be operated in parallel, serially or in a combination of both. The parallel operation mode is especially suited for screening tasks. So it is feasible to study the influence of the membrane material on the electrochemical process since different membranes can be installed in each of the electrochemical cells in the stack, which are otherwise the same. The results of the investigation can then be used to select the best membrane for the process.
"The reactor allows for a vast number of cell variations, which means that different membrane materials and electrocatalysts can be modified and tested, for example," says the researcher. The flow cells for hte GmbH are unique in the fact that their fundamental cell structure—the reactor configuration—can be further varied.
An initial screening module prototype with four electrochemical cells arranged in parallel has been delivered and is undergoing validation. We are in the progress of expanding this to 16 parallel cells. These reactors can be used in investigations into how water electrolysis can be optimized, for example. Here, it must be determined which electrocatalysts and membranes increase the efficiency of the process. Thanks to its modular design, they can also be used for other processes.
As a result, the current cell design makes it possible to cover a variety of reactor configurations—for example, with different electrode spacings—illustrating the broad range of applications. The numerous reactor configurations allow the screening platform to be adapted to different areas of application. As such, other tasks can also be considered in addition to water electrolysis, such as the production of active pharmaceutical ingredients or the decomposition of waste in wastewater treatment.
According to initial estimates, parallel testing—enabled by the new electrochemical flow cells—might accelerate the screening of catalysts by up to four times compared to classic approaches taken in long-term experiments. Over the year, the new flow cells are expected to be integrated into the hte GmbH pilot plant.
"Generally, we can make specific adaptations to our scalable electrochemical microreactors so that they can be used in different types of tasks. Electrochemistry is currently experiencing a renaissance driven by the search for green synthesis processes and efforts towards the direct use of (excessive) sustainably generated energy," summarizes Löb.
Provided by Fraunhofer-Gesellschaft