This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Evolving chemical system changes its environment under the influence of light
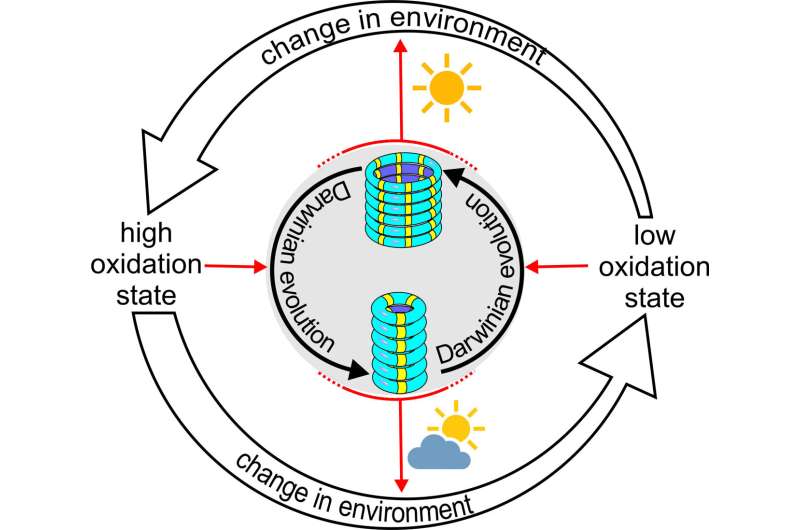
A chemical system of synthetic replicators shows the first signs of Darwinian evolution: Two different replicators compete for a common building block, and which one wins depends on the environment. As the replicators can also change their environment, ecological-evolutionary dynamics ensue. This finding shows that Darwinian principles extend beyond biology to synthetic systems.
These results, which may be used to develop new catalysts, were published by chemists from the University of Groningen (the Netherlands) in the journal Nature Chemistry on 31 August.
What is life? This question has puzzled scientists for ages. Sijbren Otto, Professor of Systems Chemistry at the University of Groningen, addresses the question by attempting to synthesize a simple form of life from scratch.
He has experimented extensively with a system of monomers, that react with each other to produce rings. These, in turn, assemble into fibers. In this process, the rings are replicated and the fibers grow and divide. Previous work showed that the fibers can also perform catalysis under the influence of light, accelerating the formation of the molecules they grow from: a primitive form of metabolism.
Adapting
In their recent study, Otto and his team focused on another key aspect of life: Darwinian evolution. They studied fibers made from self-replicating rings of two different sizes: 3-rings and 6-rings. "All rings assemble from the same monomer, for which they compete," explains Otto.
"We then placed these systems in a flow cell while adding a solution of monomers at a constant rate. At the same time, we were removing an equal amount of fluid from the cell." The scientists then watched the fibers reproduce and evolve by changing their ring size.
Replicators will only survive if they can replicate faster than the rate at which they are removed by the outflow. In this system, the two replicators exhibited different growth rates in different environments: the 3-rings grew fastest if they were in a highly oxidized environment, while the 6-rings won the competition if the environment was less oxidizing. Otto said, "We saw that the replicators could mutate into a different ring size when the oxidation state of the environment was changed. Thus, these replicators appear to be capable of adapting to a changing environment."
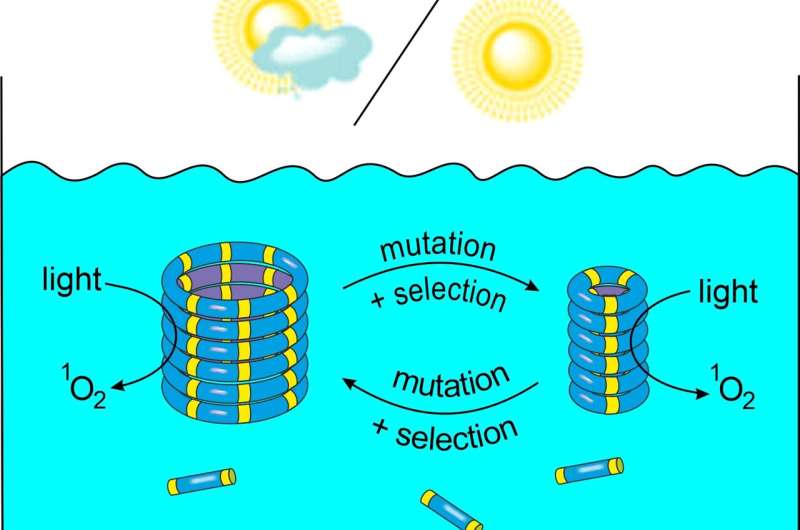
Eco-evolutionary dynamics
The researchers further developed this system by giving the replicators the ability to alter the oxidation state of their environment by themselves in response to light. Weak light conditions caused only little oxidation, allowing the 6-ring replicators to dominate. However, under strong light, the 6-ring replicators increased the oxidation level, thereby poisoning its own environment. This reduced the ability of this replicator to grow, and the mutant 3-ring fibers now took over.
"Our system is very simple, yet it shows some of the dynamics normally only seen in living systems," says Otto. "We showed how a kind of natural selection determines which type of replicator dominates, and also that these replicators can change their own environment, which, in turn, influences replicator evolution. Such eco-evolutionary dynamics are well known in biology, and it is now clear that they also extend to (our) synthetic system." But Otto does not yet call the system alive, as this would require additional features, such as the compartmentalization of the replicators in a cell-like structure.
'A limited kind of Darwinian evolution'
"However, it is interesting that Darwinian principles, which are the cornerstone of biology, can also be introduced into our synthetic system. We have replication, a metabolism, and now also a limited kind of Darwinian evolution," concludes Otto. "This is still very rudimentary, but we are keen to see if we can push our systems to become ever more life-like."
Apart from uncovering how chemical systems can transition into living ones, such systems could also harness the inventive power of Darwinian evolution to develop novel catalysts or materials, for example.
More information: Kai Liu et al, Light-driven eco-evolutionary dynamics in a synthetic replicator system, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01301-2
Journal information: Nature Chemistry
Provided by University of Groningen