This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Transforming chemistry education through an innovative experiment
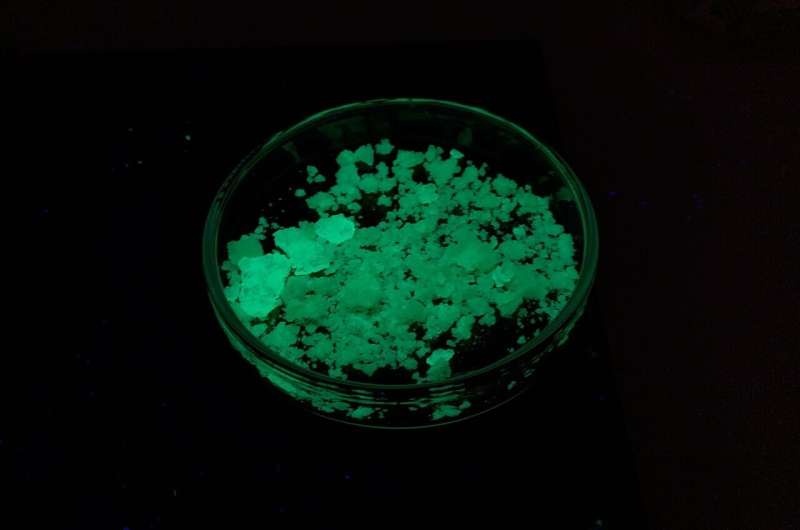
A new study conducted at the University of Chemistry and Technology has unveiled an innovative laboratory experiment that promises to ignite students' passion for chemistry and enhance their understanding of fundamental scientific principles. Led by corresponding author Jan Havlik, the research introduces a visually captivating experiment that explores the triboluminescence phenomenon through the synthesis of a manganese complex.
Triboluminescence, a fascinating phenomenon resulting from mechanical stress applied to crystals, has long intrigued scientists and educators alike. This research not only advances our understanding of triboluminescence but also transforms it into an engaging educational tool. The study's findings hold significant implications for science education, particularly in high schools and universities.
"Our research bridges the gap between theoretical knowledge and hands-on experience by offering students a simple yet enriching laboratory activity," stated Jan Havlik, the corresponding author of the study. "Through this experiment, students not only delve into the fascinating world of triboluminescence but also develop crucial laboratory skills and a deeper appreciation for the intricate properties of chemical compounds."
The experiment involves the synthesis of a manganese complex, [MnBr2(Ph3PO)2], which displays remarkable triboluminescent, fluorescent, and magnetic properties. High school students participating in the laboratory exercise successfully prepared the complex, demonstrating yields ranging from 32% to 96% of theoretical yield. Through hands-on experience, students engaged in crystal preparation, observed triboluminescence, and gained insights into fluorescence and magnetic behavior.
"By offering students the opportunity to witness and analyze triboluminescence and other properties of this unique compound, we are fostering a new generation of curious and skilled chemists," Havlik added. "This experiment is not only captivating but also safe and cost-effective, making it an ideal addition to various educational settings."
The research holds promise for diverse educational environments, including high school and university laboratories, science clubs, and public science outreach activities. The simplicity and accessibility of the experiment empower educators to inspire students' interest in chemistry and lay the foundation for a deeper understanding of scientific phenomena.
More information: Vaclav Matousek et al, Exploring Triboluminescence and Paramagnetism: A Rapid Mn Complex Synthesis for High School and Undergraduate Chemistry Laboratories, Journal of Chemical Education (2023). DOI: 10.1021/acs.jchemed.3c00372
Journal information: Journal of Chemical Education
Provided by University of Chemistry and Technology Prague