This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Development of new p38 protein inhibitors with therapeutic potential for some heart diseases
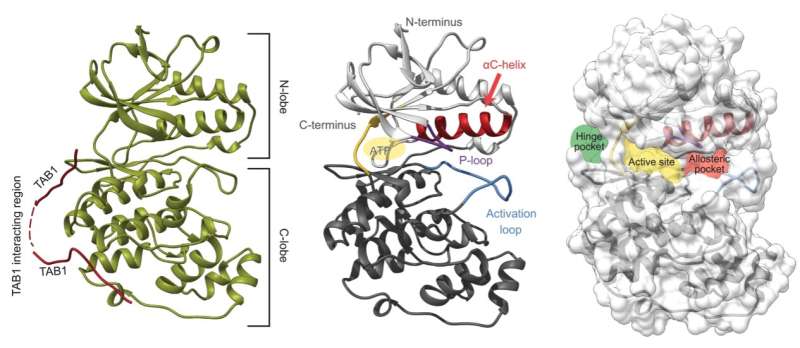
The p38 protein regulates a wide variety of cellular functions and is related to diseases such as chronic inflammation, immunological disorders, or cancer. To date, it has been difficult to find p38 inhibitors for use in clinical practice because the candidates produce toxic effects that preclude their administration at adequate therapeutic doses.
A multidisciplinary team led by Dr. Ángel R. Nebreda, Dr. María J. Macías and Dr. Modesto Orozco, all at IRB Barcelona, has developed a new type of p38 inhibitor, which preferentially impairs one of the activation pathways of this protein. In particular, these inhibitors block the self-activation (or autophosphorylation) of p38 but allow it to continue to be activated by other mechanisms.
This selective inhibition allows the p38 protein to perform many of its normal functions, thus potentially reducing the side effects associated with its total inhibition.
Specifically, the pathway blocked by the new compounds is involved in cardiac cell death caused by the lack of blood supply and subsequent restoration that occur after myocardial infarction.
The p38 self-activation pathway may also be involved in the heart damage caused by treatment with some anti-tumor chemotherapeutics.
"The selective inhibition of some of the functions of a protein as important and versatile as p38 is an innovative approach that paves the way for the development of new compounds with therapeutic potential," says Dr. Nebreda, ICREA researcher and head of Signaling and Cell Cycle lab at IRB Barcelona.
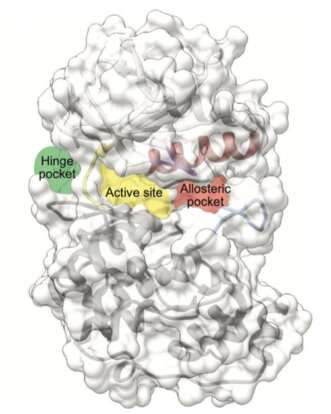
Computational modeling, biochemical and structural studies
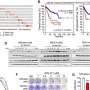
Computational techniques were used to predict protein behavior—a technology developed by Dr. Orozco's group and Nostrum Biodiscovery. Nostrum Biodiscovery is a joint spin-off of the Barcelona Supercomputing Center (BSC-CNS), IRB Barcelona, the Catalan Institution for Research and Advanced Studies (ICREA) and the University of Barcelona (UB); and collaborates with IRB Barcelona to perform a drug-discovery process. Particularly, Nostrum Biodiscovery performed hierarchical virtual screenings and in silico hit optimization studies that were key for the identification of compounds able to inhibit p38a autophosphorylation.
For the validation and characterization of these inhibitors, Dr. Nebreda's group carried out a wide range of biochemical assays, in which they analyzed more than 100 compounds.
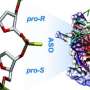
In addition, structural biology techniques that were undertaken by the group led by Dr. Macías and in collaboration with Dr. Joan Pous (from the IRB Barcelona-CSIC X-ray platform) have revealed how the inhibitors can attach to the structure of the p38 protein thus shedding light on their mechanism of action.
"The type of compounds that we have discovered is very special. They compete with the ATP molecule to bind to the active center of p38, but they do not have very high affinity. So as soon as the protein is activated by an external factor, ATP displaces the inhibitor and p38 can exert its normal functions," explains Dr. Lorena González, first author of the study, who carried out this project as part of her thesis at IRB Barcelona.
The team has started collaborating with Dr. Antonio Rodríguez-Sinovas, a specialist in cardiovascular diseases at the Vall d'Hebron Research Institute (VHIR), to validate the possible therapeutic potential of the inhibitors in models of cardiotoxicity.
More information: Lorena González et al, Characterization of p38α autophosphorylation inhibitors that target the non-canonical activation pathway, Nature Communications (2023). DOI: 10.1038/s41467-023-39051-x
Journal information: Nature Communications