This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Laser-controlled intracellular flows in temperature-sensitive biological cells
Micromanipulation techniques are widely adopted in materials science, colloidal physics and life sciences for various applications, ranging from nanostructure assembly and particle trapping to spatio-temporal analysis of cell organization. Introduced optically induced thermoviscous flows, i.e. focused-light-induced cytoplasmic streaming (FLUCS), can manipulate the cytoplasm in cells and developing embryos.
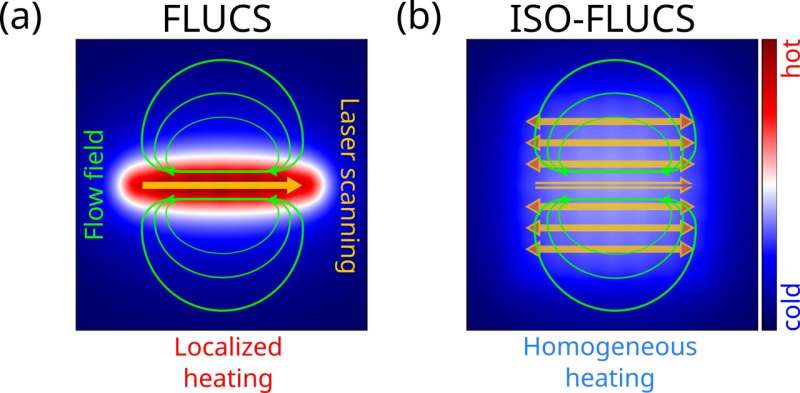
Thermoviscous flows arise from the complex interplay of thermal expansion and temperature-induced viscosity changes when repeatedly moving a heating point stimulus through a thin fluid film. Specifically, the localized heating generated by scanning the IR-laser spot through the sample induces a small local change in the density and viscosity of the fluid, resulting in a locally compressible fluid flow with a net transport of tracers.
Although FLUCS has the advantage of generating directional flows with highly reduced invasiveness, samples still experience some temperature modulation. This could affect highly heat-sensitive systems, such as thermosensitive mammalian cells.
In a new paper published in eLight, a team of scientists led by Professor Moritz Kreysing from Karlsruhe Institute of Technology developed nearly isothermal scan sequences that could open a path for new soft matter and biomedicine avenues.
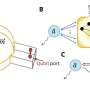
The research team showed that a step-by-step optical strategy could disentangle laser-induced flows and heating. They use previously disregarded degrees of freedom that accompany the optical generation of flow fields. The temperature distribution is homogenized over the region of interest (ROI) by introducing additional counter-directed paths, symmetrically arranged around the desired trajectory.
In particular, the team has exploited symmetry relations on up to three distinct time scales. It led to locally homogeneous temperature distributions while inducing directional flows with flow lines that are largely unaltered compared to standard FLUCS. Additionally, the sample is cooled to the required temperature with an external Peltier cooling system.
The researchers have demonstrated that this technology, called isothermal-FLUCS (ISO-FLUCS), is associated with at least a 10-fold reduction in heating while achieving thermoviscous flows whose magnitudes well exceed endogenous streaming in Caenorhabditis elegans zygotes.
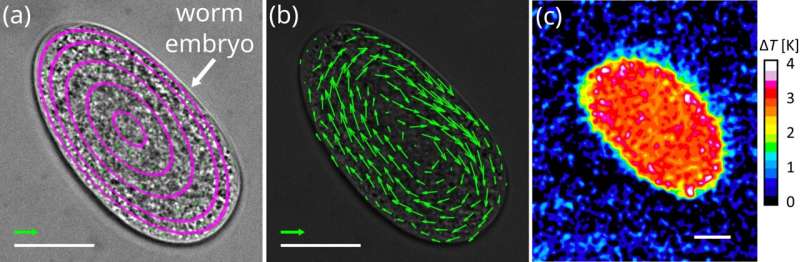
Given its drastically reduced heating impact while retaining FLUCS' main features, the researchers believe that ISO-FLUCS will become the new standard for these optical micromanipulations in highly temperature-sensitive systems in biology and materials science.
ISO-FLUCS can drastically improve the temperature homogeneity inside samples (standard deviation reduced up to 20 times). These results were achieved by implementing three new ingredients to the well-established FLUCS: (i) flow and heating disentanglement, (ii) multi-timescale scan sequence symmetrization, and (iii) residual high-order field cancelation.
Neutral scan lines were conveniently included in the pattern to flatten the temperature gradient in the region of interest (hundreds of µm2). Spatiotemporally symmetric scan sequences ensured predictable and highly symmetric thermoviscous flow fields. The heating level in conventional FLUCS was previously demonstrated to be tolerable in sensitive biological systems, such as developing C. elegans zygotes.
The clean disentanglement of flows and local heating that ISO-FLUCS offers will not only become the new unimpeachable standard for precise flow manipulation inside a living specimen. It also lays the foundations to specifically address different classes of physical stimuli relating to mechanics and forces and temperature-dependent biochemistry of rate equations. This will advance flow-driven microrheology by eliminating any temperature-dependent material responses caused by the measurements.
As ISO-FLUCS operates over a very narrow temperature range that can be extremely well controlled, it is also expected to find wide use in studying temperature-sensitive polymeric or particulate hydrogels. The accurate determination of the sol-gel transition is of utmost importance for understanding emerging properties on the macroscale. The fine temperature control in ISO-FLUCS can also be used to investigate the spinodal decomposition of many systems that exhibit a high propensity towards phase separation.
The research team believe that ISO-FLUCS will replace FLUCS in becoming the new standard for such laser-induced optical micromanipulations in the most sensitive samples. Additionally, ISO-FLUCS resonates with a rapidly growing community to harness the power of temperature stimuli in manipulating colloidal and living systems on the micro- and nanoscale. In the future, the team foresees ISO-FLUCS paving the way to medical use cases, such as laser-assisted human reproductive medicine.
More information: Antonio Minopoli et al, ISO-FLUCS: symmetrization of optofluidic manipulations in quasi-isothermal micro-environments, eLight (2023). DOI: 10.1186/s43593-023-00049-z
Provided by Chinese Academy of Sciences