This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Building models to predict interactions in plant microbiomes
Plants, animals, and humans are all home to numerous microorganisms such as bacteria and fungi. These form complex communities that have a profound impact on the health of their host. One notable microbiome is that of the human gut, which helps digest our food and protect us against pathogens.
Plants are also host to microbial communities on their roots and leaves. These communities can promote growth and keep off harmful bacteria. Plant microbiomes therefore have the potential to make agriculture more sustainable. However, we currently only have a rudimentary understanding of the interspecies interactions that shape these microbial communities.
Why is it that these communities tend to be populated only by certain kinds of microbes and not others? "We already knew that leaf microbiomes weren't just some random collections of microbes," says Julia Vorholt, Professor of Microbiology at ETH Zurich. "But the rules that determine how these communities form and what mechanisms shape their makeup remained to be found."
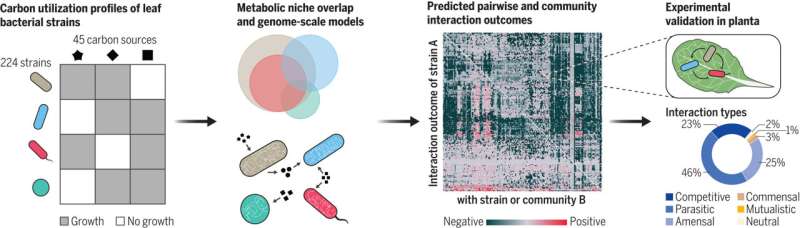
Now, a team of researchers led by Vorholt has identified just such an organizing principle for the bacteria that live on the leaves of the model plant Arabidopsis thaliana (thale cress). The researchers have developed a set of models that use the nutrient preferences and metabolic abilities of individual bacterial strains to predict how these leaf surface microbes compete or cooperate with each other, thereby helping us better understand the nature of the resulting microbiome.
The research team's study, which was carried out in collaboration with colleagues at EPFL, has been published in the latest issue of the journal Science.
Resource competition leads to distinct interactions
As part of a previous work, Vorholt's group had already shown that the microbial communities found on plant leaves were remarkably similar. "The consistent composition of these communities points to an underlying mechanism that controls how the leaf microbiome is created," Vorholt says.
Martin Schäfer, a postdoc in Vorholt's group and co-lead author on the study, explains that "since all bacteria ultimately depend on organic molecules as food, we asked whether we could predict the way they interact by knowing which food molecules they can metabolize."
Alan Pacheco, also co-lead author, adds, "in a competitive environment, food niches could lead to stable coexistence and collaboration, with the microbes interacting for mutual advantage by exchanging resources."
The guiding question posed by Vorholt and her team is: Can the use the metabolic capabilities of different bacteria to understand how the leaf microbiome takes shape?
Carbon profiles reveal resource competition
To answer this question, the researchers began by testing the ability of more than 200 representative strains of bacteria from Arabidopsis thaliana leaves to grow using 45 different carbon sources. Using these carbon profiles, they determined that there was extensive overlap between the strains' food niches. This indicates that there is fierce competition for resources.
The researchers then used these carbon profiles to build a set of reliable metabolic models for all bacterial strains, and simulated interactions between more than 17,500 pairs of bacteria. Consistent with the extensive overlap in food niches, the simulations showed a marked dominance of negative interactions: when competition causes the population of at least one of the two strains to decrease.
Sidestepping competition through cooperation
Despite this prevalence of competition, the metabolic models also predicted positive interactions. A closer analysis revealed that these cooperative interactions can be traced back to the exchange of organic and amino acids. The study's authors carried out plant experiments to test the models' predictions and were able to confirm them to an accuracy of 89 percent.
The accuracy of the models came as a surprise even to the researchers themselves: "The high degree of reliability suggests that our initial assumptions about the importance of metabolic characteristics were correct," Pacheco says.
Harnessing microbiomes for application
"What's great about our models is that they also work in reverse," Vorholt says, "in that they can be used to identify mechanisms that trigger certain interaction patterns." This paves the way for targeted microbiome design, which is a key prerequisite for downstream applications in agriculture.
Currently, seed companies and agricultural chemical producers use a process of trial and error to search for microbes that sustainably support crop protection. The team's findings are therefore relevant not only for fundamental research, but also for applications in microbiome design for agriculture.
Vorholt is Co-Director of the Swiss National Centre of Competence in Research (NCCR) Microbiomes. Her team's current study furthers the research by a network of 20 groups, whose aim is to understand microbiomes—from plants to humans—so that their vast potential for health, agriculture, and environment can be realized.
This can be achieved by, for example, supplementing unbalanced communities with the right microbe, removing certain species, or even treating diseases with combinations of bacteria with special functions. Predictive models will play a key role in this goal.
More information: Martin Schäfer et al, Metabolic interaction models recapitulate leaf microbiota ecology, Science (2023). DOI: 10.1126/science.adf5121
Journal information: Science
Provided by ETH Zurich