This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study offers insights into how cells reverse their decision to divide
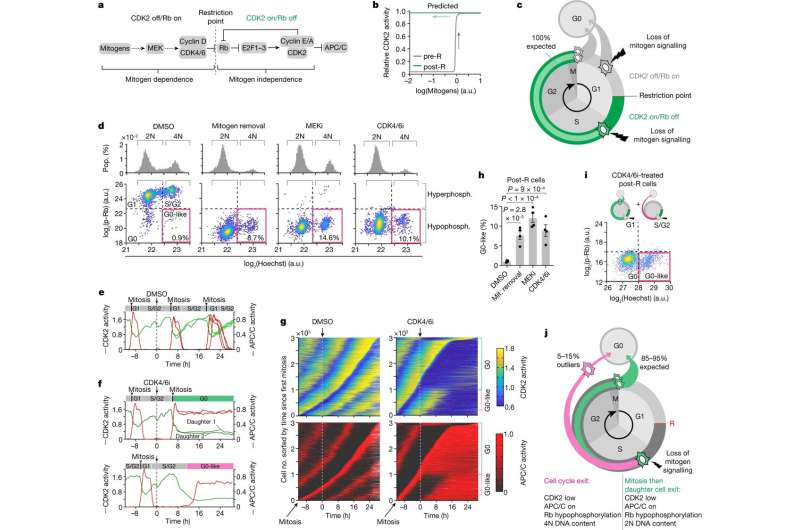
A new study suggests that cells preparing to divide can reverse this process and return to a resting state, challenging long-held beliefs about cell division. If interrupted early in their preparation to divide, cells were able to halt the division process, known as mitosis.
The finding, led by researchers at the National Cancer Institute (NCI), part of the National Institutes of Health, and reported July 5, 2023, in Nature, could point toward more effective treatments to interrupt the process by which cancer cells divide quickly and spread.
When cells receive growth-promoting signals, called mitogens, they enter the cell cycle—synthesize new copies of their DNA in a series of steps that culminate in cell division. Scientists have long thought that the preparatory stage of this cycle includes a point after which cells cannot halt the process. Researchers believed that after this "point of no return," growth signals are no longer needed to drive cells to divide.
In the new study, scientists at NCI's Center for Cancer Research captured videos of thousands of cells undergoing mitosis and watched what happened to those cells when mitogens were withdrawn. About 15% of the cells exited the cell cycle and returned to a resting state.
What those cells had in common was that they hadn't been as far along as others in the cycle when they stopped receiving growth-promoting signals. In experiments with many different kinds of cells, researchers found that all types of cells were capable of exiting the cell cycle if it was early enough.
Drugs that inhibit the cell cycle regulators CDK4 and CDK6, such as the breast cancer drug palbociclib (Ibrance), likely interrupt cells' progression through the cell cycle differently than previously thought, the researchers said. They are now looking at whether they can take advantage of this new molecular mechanism to design a more durable therapy by combining CDK4 and CDK6 inhibitors with traditional chemotherapy drugs that induce DNA damage.
More information: Steven Cappell, Loss of CDK4/6 activity in S/G2 phase leads to cell cycle reversal, Nature (2023). DOI: 10.1038/s41586-023-06274-3. www.nature.com/articles/s41586-023-06274-3
Journal information: Nature
Provided by National Institutes of Health