This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Extracellular cytochrome nanowires appear to be ubiquitous in microbes
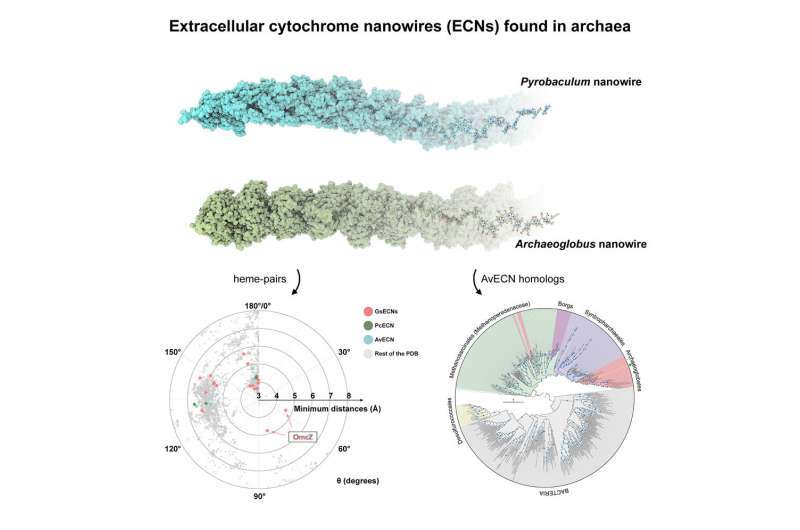
A Geobacter bacteria isolated from a contaminated ditch in Oklahoma has an unusual appendage—a long extracellular nanowire that can conduct electricity. The electron transport chain in this nanowire carries electrons from the bacteria to an insoluble external electron acceptor to help the microbe make energy. Such long-range electron transfers at a micron-scale are thought to have played an important role in microbial metabolism on the early Earth.
Now Fengbin "Jerry" Wang, Ph.D., of the University of Alabama at Birmingham and colleagues in Paris and Virginia report that such nanowires, composed of a long chain of cytochrome proteins, appear to be ubiquitous in prokaryotic microbes—both in bacteria and in archaea.
The archaea microbes are the second prokaryotic domain of life besides bacteria, and archaea are thought to be an ancient intermediate group between the bacteria and the more advanced eukaryotic cells found in lifeforms like animals and plants.
Besides strongly suggesting that these extracellular cytochrome nanowires, or ECNs, are ubiquitous in many prokaryotes that rely on long-range electron transfer for metabolism, co-corresponding authors Wang, Mart Krupovic, Ph.D., Institut Pasteur, Paris, and Edward H. Egelman, Ph.D., University of Virginia School of Medicine, Charlottesville, Virginia, say their findings establish that the extracellular cytochrome filaments found in Geobacter sulfurreducens are not unique.
Their research, published in the journal Cell, used bioinformatics and molecular structure determination through cryo-electron microscopy.
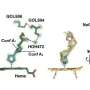
G. sulfurreducens has three ECNs, each one a long chain made from one of three different c-type cytochromes. Each individual c-type cytochrome molecule contains multiple hemes, the iron-containing porphyrins that attach to the cytochrome and act in electron transfer. Heme is more commonly known as the oxygen carrier in the hemoglobin of red blood cells.
The researchers looked at genetic datasets of archaebacterial species predicted to have multi-heme c-type cytochromes, and they used multiple filters to identify cytochromes that might form ECNs. The protein had to: 1) have a "signal peptide sequence" that helps transport the protein to the membrane of the microbe, 2) lack transmembrane regions that would have to insert into cell's membrane, 3) be encoded with other multi-heme c-type cytochromes in the same strain, and 4) lack sequence homology to existing known protein folds, meaning the cytochromes were predicted to have as yet unseen three-dimensional secondary structures.
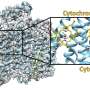
From these criteria, they tested three extreme-thermophile ECN candidates, and they were able to grow ECNs—as identified by cryo-electron microscopy—from two of the species belonging to different phyla. The researchers also used cryo-electron microscopy to determine the molecular structures of the two ECNs, at resolutions of 3.8 and 4.1 Ångstroms.
One ECN was from Pyrobaculum calidifontis, which had been isolated from a terrestrial hot spring in the Philippines. In the lab P. calidifontis is grown at a temperature of 194° F. The other ECN was from Archaeoglobus veneficus, isolated from the Snake Pit deep-sea hydrothermal vent site, 11,500 feet deep on the mid-Atlantic Ocean Ridge. In the lab A. veneficus is grown at 167° F.
The researchers were able to identify which specific c-type cytochrome, out of a number of different c-type cytochromes in each microbe's genome, was used to grow its ECN. These nanowires were designated PcECN, the P. calidifontis ECN, and AvECN, the A. veneficus ECN. PcECN T and AvECN had three striking similarities to two of the ECNs in G. sulfurreducens.
First, all four ECNs have insulated hemes, meaning protein surrounds the internal hemes like insulation on a copper wire, so the hemes are protected from solvent interaction. Second, each cytochrome unit in the four ECNs has four heme sites. Third, the three-dimensional heme stacking pattern in all four ECNs were remarkably similar, despite the structurally unrelated cytochromes of the different ECNs showing no similarity in protein folds. This suggested that there is an optimized mode of heme stacking for a solvent inaccessible ECNs.
When the researchers searched the Protein Data Bank, they were surprised to discover that this heme stacking is also present in enzymes that transfer electrons over shorter distances, despite the functional divergence among them and no recognizable fold similarity.
These proteins included nitrite reductase, sulfite reductase, NrfB, photosynthetic cytochrome c552, hydroxylamine oxidoreductase, Mtr complex and three types of oxidoreductases. Thus, the four-heme array pattern is ubiquitous in nature, suggesting this is an evolutionarily optimized heme-orientation for efficient electron transfer.
The researchers found that homologs of the ECN cytochromes—meaning other proteins with a common evolutionary origin—are widespread in archaea. Specifically, homologs of PcECN were detected in the archaeal order Desulfurococcales, while homologs of AvECN were distributed more broadly.
AvECN homologs were found in other hyperthermophilic members of order Archaeoglobales, and also among alkane degrading archaea of the order Syntrophoarchaeales, methane oxidizers of the family Methanoperedenaceae and in multiple extremely large megaplasmids called Borgs. Borgs are associated with methane-oxidizing Methanoperedens archaea.
Wang and colleagues showed the evolutionary relationships among these homologs. They also found that the amino acid motifs involved in heme attachment and coordination are well conserved in Archaeoglobales, Syntrophoarchaeales and Methanoperedenaceae, as well as Borgs, which suggests these ecologically important archaea can form structurally similar ECNs.
Other bioinformatics analysis suggested that the AvECN protein family and a c552-family cytochrome from a purple sulfur photosynthetic bacteria share a common ancestor in their evolution.
More information: Diana P. Baquero et al, Extracellular cytochrome nanowires appear to be ubiquitous in prokaryotes, Cell (2023). DOI: 10.1016/j.cell.2023.05.012
Journal information: Cell
Provided by University of Alabama at Birmingham