This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New study reveals a hidden mechanism for controlling a cell's molecules
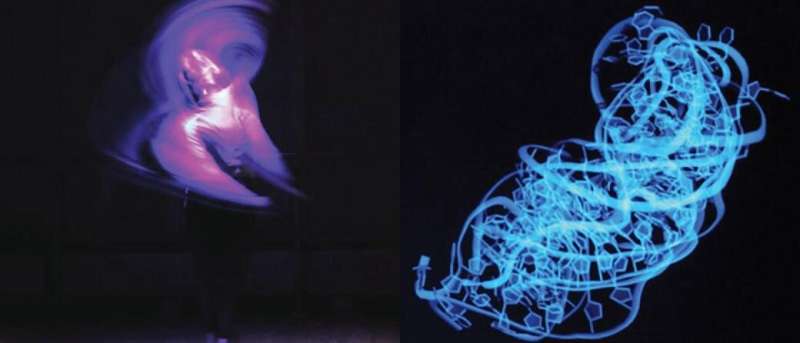
Fundamentally, life comes down to molecules contacting and binding to other molecules.
"Everything you see in biology is the consequence of interactions between molecules, so understanding how two molecules stick together is a fundamental question, it's like the basic programming language of biology," says Hashim Al-Hashimi, Ph.D., the Roy and Diana Vagelos Professor of Biochemistry and Molecular Biophysics at Columbia University Vagelos College of Physicians and Surgeons.
Predicting just how two molecules might interact with each other remains one of the most important challenges in biology and would accelerate the design of new drugs, but there's a problem with how molecular interactions are currently studied.
The most popular techniques for studying molecular interactions yield static images, showing molecules' shapes before and after binding each other.
But as Al-Hashimi explains, "no molecule is static, each one can adopt slightly different shapes, creating an ensemble of structures that interconvert on time scales from picoseconds to seconds in solution. When you take an image, you're just revealing the most dominant structure, the structure the molecule spends more time in than others."
How a molecule is like a tall person in a coach-class seat
Probing dominant structures can only take researchers so far, because to bind a specific partner, a biomolecule such as RNA, DNA, or protein must usually adopt one of its less common structures, like a tall person stooping and squatting to fit into a coach-class airline seat. And similar to how a tall person must exert energy to get into that uncomfortable position, energy is required to get a molecule into its binding configuration. Al-Hashimi compares this energy cost to a tax the molecules have to pay in order to bind.
Al-Hashimi's graduate student Megan Ken reasoned that if researchers could calculate that tax, they could determine precisely how readily any two molecules would bind. To do that, Ken and Al-Hashimi used nuclear magnetic resonance spectroscopy, adapting the technique to reveal the entire ensemble of structures of a specific molecule in solution. The probability of forming a particular shape is a measure of the tax needed to form that shape.
As a test case, they focused on the transactivation response element (TAR) from HIV, an RNA sequence that binds a viral protein called Tat to start the virus's replication cycle.
The team, collaborating with Dan Herschlag at Stanford, Bryan Cullen at Duke and Ursula Schulze-Gahmen at the Gladstone Institute of Virology, tested the effect of different mutations to TAR on both the molecule's propensity to form biologically active structures and its function in cells.
Some mutations were like a tax rebate and raised the propensity of TAR to adopt its biologically active structures, while other modifications raised the tax and decreased the odds that TAR would adopt a biologically active structure. The research was published May 17 in Nature in a paper titled "RNA conformational propensities determine cellular activity."
Hidden mechanism may lead to new drugs
"What we found was a hidden mechanism for controlling the activity of a molecule in a cell," says Al-Hashimi. By altering the propensity of TAR to form biologically active states—by changing the tax rate—the researchers could fine-tune the molecule's activity in cells.
Al-Hashimi adds that this approach can also be applied to other RNA-protein and protein-protein interactions and points to a new strategy for designing drugs.
His team is currently looking for drugs that can prevent TAR from adopting its active state and thereby prevent HIV from replicating.
"If successful, the approach could be a new way to develop drugs for a wide variety of diseases."
More information: Megan L. Ken et al, RNA conformational propensities determine cellular activity, Nature (2023). DOI: 10.1038/s41586-023-06080-x
Journal information: Nature
Provided by Columbia University Irving Medical Center