This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
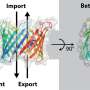
Researchers reveal architecture of the ESCPE-1 membrane coat
A common activity in our daily household chores is separating paper, glass, cans, and plastic to deposit them in the appropriate containers. Through recycling, we can reduce resource consumption, save energy, and minimize waste. Similarly, our cells recycle many of their components to achieve the same benefits.
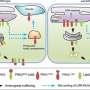
In the realm of cellular biology, the recycling of membrane proteins plays a vital role in maintaining cellular function and equilibrium. A remarkable protein complex called endosomal sorting complex for promoting exit 1 (ESCPE-1) has emerged as a key player in this process.
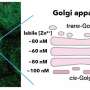
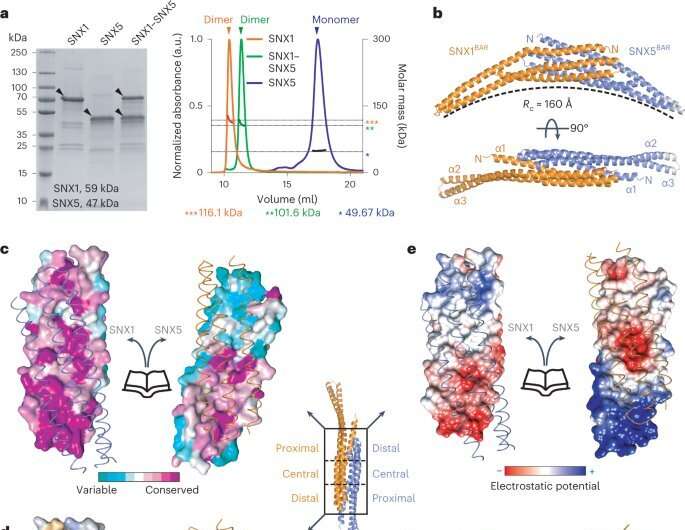
By rescuing transmembrane proteins from the endolysosomal pathway, ESCPE-1 ensures their safe transport to the trans-golgi network and ultimately to the plasma membrane. While the importance of ESCPE-1 in recycling is well-established, the underlying mechanisms governing its function have remained elusive. Recent research has started to shed light on the intricate workings of ESCPE-1 and its role in tubule-based endosomal sorting. The research was first published online in the journal Nature Structural & Molecular Biology.
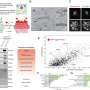
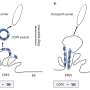
A multidisciplinary research team coordinated by Aitor Hierro, Ikerbasque lead researcher at CIC bioGUNE, Biofisika Institute, NIH (National Institutes of Health, U.S.), BSC (Barcelona Supercomputing Center), and ICVV (Institute of Vine and Wine Sciences) has revealed the structure of ESCPE-1 (Endosomal Sorting Complex Promoting Exit 1), which is responsible for transporting and reusing over 60 different proteins. This research provides valuable insights into the intricate mechanisms of tubular-based cargo sorting mediated by ESCPE-1.
By elucidating the relationships between membrane interactions, cargo recognition, and coat formation, the study enhances our understanding of membrane protein recycling and its role in cellular processes.
"Many of the proteins transported and reused by this cellular machinery for protein recycling are cell receptors involved in cell growth and proliferation, and they appear dysregulated in different types of cancer. In this study, we have revealed the organization of ESCPE-1 at the atomic level and how the receptors to be recycled contribute to their own transport. Going back to the analogy of paper, glass, cans, and plastic, it is like discovering the mechanism of selective collection for one of these containers," explains Aitor Hierro.
The work, which has been carried out over the past five years, has utilized two of the most relevant techniques in structural biology: X-ray crystallography and cryo-electron microscopy. Both techniques require large infrastructures like those found at CIC bioGUNE and BREM (Basque Resource for Electron Microscopy) at the Biofisika Institute, which together have successfully addressed this study.
More information: Carlos Lopez-Robles et al, Architecture of the ESCPE-1 membrane coat, Nature Structural & Molecular Biology (2023). DOI: 10.1038/s41594-023-01014-7
Journal information: Nature Structural & Molecular Biology
Provided by CIC bioGUNE