This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New pathway discovered for RNA degradation in iron-rich environments
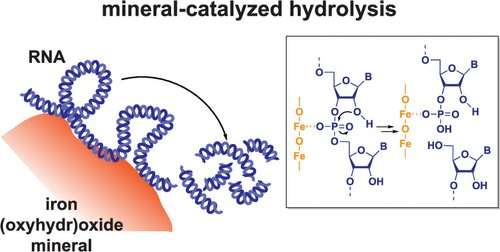
RNA, an essential biomolecule for life, has been used in environmental applications including monitoring microbial communities, developing pesticides, and quantifying the abundance of pathogenic viruses, such as SARS-CoV-2, in water and wastewater systems. Understanding how quickly RNA breaks down in given conditions is critical to harnessing the molecule in these and other emerging technologies.
According to a new study by researchers working with Kimberly Parker, assistant professor of energy, environmental & chemical engineering in the McKelvey School of Engineering at Washington University in St. Louis, RNA can undergo rapid hydrolysis when adsorbed into iron oxide minerals. This discovery unveils a previously unknown abiotic pathway for RNA degradation and sheds light on biogeochemical processes and environmental system dynamics. The results were published May 22 in Environmental Science & Technology.
"This is the first abiotic process we've found that causes RNA degradation in the environment on timescales that can compete with biotic degradation," Parker said. "Instead of depending on biological agents like enzymes or microbes to break down RNA molecules, we found that RNA degradation catalyzed by minerals happens relatively quickly regardless of the biological context. This could be an important limit on how long RNA persists in the environment."
First author Ke Zhang conducted the research in Parker's lab while earning a doctorate in environmental engineering at WashU in 2022. Zhang found that RNA undergoes rapid hydrolysis on the timescale of hours when adsorbed to iron oxide minerals such as goethite and hematite. This hydrolysis process is uniquely facilitated by the presence of iron in the minerals, which chemically accelerates the structural breakdown of the RNA molecule. This finding challenges scientists' previous assumptions about the environmental factors affecting RNA degradation, particularly in iron-rich soils and sediments, which account for approximately 10% of global ice-free land.
"This process could provide an important limit on how long RNA hangs around in the environment, but there are certain conditions that can block this breakdown pathway," Parker said. "While we measured the reaction timescales and determined the reaction products in this research, we need to develop more insights into the reaction mechanism in the future. Understanding the mechanisms as well as timescales of RNA degradation is crucial for accurately interpreting relative amounts of DNA versus RNA, studying viruses and pesticides, and even exploring the origin of life."
More information: Ke Zhang et al, RNA Hydrolysis at Mineral–Water Interfaces, Environmental Science & Technology (2023). DOI: 10.1021/acs.est.3c01407
Journal information: Environmental Science & Technology
Provided by Washington University in St. Louis