This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Molecular signals key to malaria parasite's development, shows study
A key developmental step in the life cycle of the most virulent species of malaria parasite depends in part on a series of molecular signals, detailed in a new study led by researchers at Weill Cornell Medicine. The findings may help scientists develop new ways to prevent malaria from spreading.
The parasite, Plasmodium falciparum (P. falciparum), can spread from infected humans back to mosquitoes, completing their transmission cycle, only after they change from the asexual form that is responsible for the disease into sexual forms known as gametocytes. Scientists know that gametocyte formation is triggered when local levels of a lipid-related molecule called lysophosphatidylcholine (lysoPC) are low, as occurs in human bone marrow. But until now, they haven't understood why this happens.
In the study, published June 5 in Nature Microbiology, the researchers showed for the first time how low lysoPC leads to the depletion of a key molecule needed to suppress the genes that trigger gametocyte formation.
"Knowing in detail how P. falciparum gametocyte formation is regulated could reveal ways to block this process and thereby interrupt transmission," said study senior author Björn Kafsack, associate professor of microbiology and immunology at Weill Cornell Medicine.
P. falciparum, a single-celled parasite, is one of five malaria parasite species that routinely infect humans, but it is by far the worst, accounting for virtually all of the world's roughly 600,000 malaria deaths each year. Scientists study P. falciparum biology in the hope of finding vulnerabilities that can be exploited with new medicines or other interventions.
A notable feature of P. falciparum is that unlike other human-infecting malaria parasites, it can infect not only immature red blood cells in bone marrow, but also mature red blood cells that are already circulating in the blood. However, producing and releasing gametocytes from circulating red blood cells would be problematic for the parasite, as they would be released in relatively immature forms that are efficiently filtered out by the spleen. P. falciparum instead produces gametocytes mainly in the bone marrow, where they tend to be sequestered until they are more mature and aren't so easily cleared by the spleen.
How does the parasite sense that it is in bone marrow rather than in the circulation? In 2017, another team of researchers discovered a big clue: lysoPC effectively suppresses P. falciparum's ability to form gametocytes. Since lysoPC is abundant in most human tissues but not in bone marrow, gametocyte production is preferentially unsuppressed in bone marrow.
In the new study, Kafsack and his team—including study first author Chantal Harris, who performed most of the experiments while a Ph.D. student in the Kafsack lab—set out to find the molecular details of how lysoPC scarcity leads to gametocyte production.
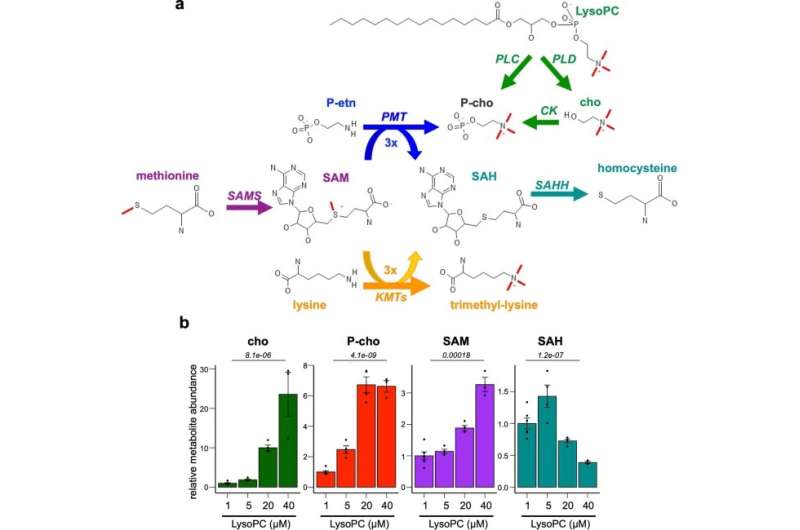
Their work uncovered what appears to be the critical pathway. P. falciparum normally uses lysoPC and related molecules to make phosphatidylcholine, an essential component of its cell membranes. When these precursor molecules are not available sufficiently in host cells, P. falciparum synthesizes phosphatidylcholine from smaller, more available chemical building blocks.
This synthesis process effectively consumes another molecule called S-adenosylmethionine (SAM)—which, the team discovered, is also needed for the process that suppresses P. falciparum's sexual differentiation genes.
"Low lysoPC levels ultimately allows gametocyte formation by removing a brake on the expression of the genes needed to make that switch," said Harris, now a postdoctoral associate at Genentech.
Although targeting this pathway to keep gametocyte production suppressed probably wouldn't alleviate illness in infected people, it would block transmission back to mosquitoes, thereby limiting local spread. Kafsack envisions future medicines that would be used to achieve this goal in combination with standard malaria treatments, though he notes that the issue of the best element in the pathway to target with drugs has yet to be resolved.
More information: Chantal T. Harris et al, Sexual differentiation in human malaria parasites is regulated by competition between phospholipid metabolism and histone methylation, Nature Microbiology (2023). DOI: 10.1038/s41564-023-01396-w
Journal information: Nature Microbiology
Provided by Cornell University