This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Research offers insights into redox-independent cellular stress response
Cellular stress, or oxidative stress, occurs when there is a buildup of reactive oxygen species (ROS), which interferes with cellular mechanisms and can even cause damage to proteins, lipids, and DNA. Due to their destructive nature, all cells have robust mechanisms in place to remove ROS and reduce oxidative stress. One such mechanism is the nuclear factor erythroid 2-related factor 2 (NRF2)-mediated stress response, where NRF2 is a master transcription factor that aids in reducing oxidative stress.
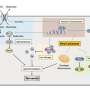
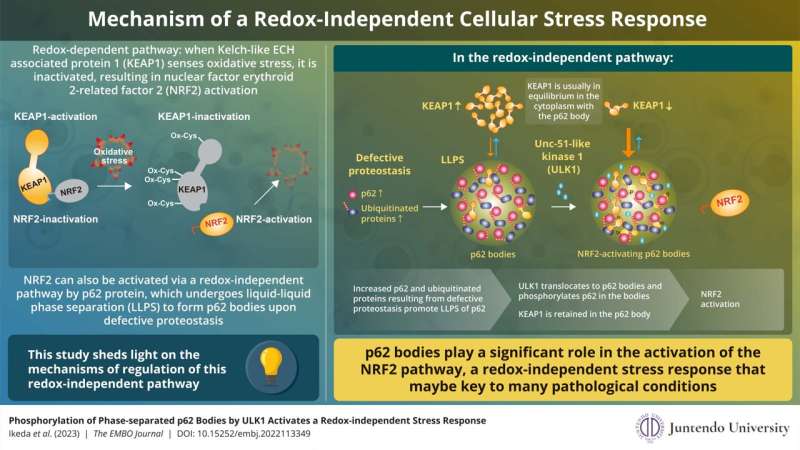
Much is known about the redox-dependent activation of NRF2 and its subsequent role in stress response. In this pathway, Kelch-like ECH-associated protein 1 (KEAP1) senses oxidative stress in the cell through oxidation of its specific cysteine residues. This oxidation causes conformational changes in KEAP1, which, in turn, loses the ability to suppress NRF2. As a result, NRF2 is stabilized and induces a series of genes encoding anti-oxidative proteins that reduce and remove oxidative stress caused by ROS.
NRF2 can also be activated in a redox-independent manner. This activation involves p62 protein, which undergoes liquid-liquid phase separation to form p62 bodies when it binds to ubiquitinated proteins upon defective proteostasis. However, the precise mechanism of NRF2 regulation through p62 bodies had hitherto remained largely unknown.
Now, researchers in Japan have found how p62 bodies control redox-independent NRF2 activation. They conducted a study that was conceived by Professor Masaaki Komatsu and Associate Professor Yoshinobu Ichimura from Juntendo University School of Medicine and Dr. Nobuo N. Noda from Hokkaido University. "We have reported in a previous study that phosphorylation of p62 inhibits the binding of KEAP1 to NRF2 competitively, thereby disabling the NRF2-repressive function of KEAP1. However, the regulatory mechanism and the physiological functions in vivo remain largely unclear. This is important as the accumulation of phosphorylated p62 has been found to cause many intractable diseases," explains Prof. Komatsu when asked about the team's motivation for pursuing the research. Their findings have been published in The EMBO Journal.
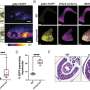
Using advanced techniques like high-speed atomic force microscopy, fluorescence recovery after photobleaching, and fluorescence loss in photobleaching, the team conducted experiments that included those performed outside a living organism (in vitro) and those using cells and mice (in vivo) to thoroughly profile protein-protein interactions, cellular localization of specific components, and the effects of LLPS-induced p62 phosphorylation, during redox-independent NRF2 activation.
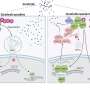
Summarizing the main findings of their study, Dr. Ichimura explains, "We found that ULK1, a protein kinase, translocates to p62 bodies and then phosphorylates p62 within the bodies. The resulting phosphorylated p62 bodies retain KEAP1 within them, which drives the activation of NRF2."
KEAP1 usually cycles in and out of p62 bodies; however, phosphorylated p62 tightly binds to KEAP1, which causes KEAP1 to be retained and sequestered within the p62 body. This leads to activation of even more NRF2. The p62 bodies are degraded by autophagy, and this may contribute to shutdown of this pathway. The current study extends the scope of the antioxidative stress response and provides new insights into the role of phase separation in the process.
The importance of this redox-independent NRF2 activation was tested using phosphomimetic p62 knock-in mouse models where hyperactivation of NRF2 by extensive phosphorylation resulted in hyperkeratosis—a growth defect causing the outer layer of the stomach and esophageal lining to thicken, which in turn caused stunted growth due to malnutrition.
Prof. Komatsu is confident that his team has laid the foundation for future work to probe deeper into the mechanism and regulation of redox-independent stress responses. He says, "Whether redox-dependent or independent, NRF2 activation is an important biological defense system. Understanding its regulatory mechanisms is crucial as its persistent activation leads to inordinate defense responses, like excessive keratinization.
"Our study is the first scientific validation of the physiological significance for redox-independent NRF2 activation. In the case of redox-independent stress responses, activation of NRF2 is very likely regulated by phosphorylation, dephosphorylation, and autophagic degradation of p62 bodies. p62 bodies have been found to accumulate in affected cells in patients with liver disorders, neurodegenerative diseases, and cancers. Thus, research such as ours will be useful in elucidating the pathogenesis of and developing improved therapies for p62-, NRF2-, and autophagy-related diseases."
More information: Ryo Ikeda et al, Phosphorylation of phase‐separated p62 bodies by ULK1 activates a redox‐independent stress response, The EMBO Journal (2023). DOI: 10.15252/embj.2022113349
Journal information: EMBO Journal
Provided by Juntendo University Research Promotion Center