This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Behind the formation and protection of microtubules
Cellular life hinges on a network of hollow cables called microtubules dynamically lengthening and shortening according to the needs of the moment. During cell division, for instance, these cables latch onto chromosomes and retract—yanking chromosomes to either end of the cell to ensure that each daughter cell receives an equitable share of genetic information. In addition to regulating the dynamics of microtubules, the cell also regulates the precise timing and location of microtubule formation. There's little room for error.
Now, a new study sheds light on how the formation of human microtubules drives cell division. The paper, published in the Journal of Cell Biology, describes the inner workings of the γ-Tubulin Ring Complex (γ-TuRC), an assembly of proteins responsible for nucleating and stabilizing microtubules. The findings clarify the γ-TuRC's mechanism, and may inform researchers studying γ-TuRC mutations and associated diseases.
"We were able to characterize the γ-TuRC's capping activity, and explore its role in cell division," says Adi Berman, a graduate research fellow in the laboratory of Tarun Kapoor at the Rockefeller University. "The more we learn about what this complex does and how it does it, the more answers we might be able to find about how the γ-TuRC relates to human diseases."
A seed and a cap
The lifecycle of a microtubule typically begins when protein dimers, composed of alpha and beta tubulin, interact to form long tubular polymers. But that process takes time that the cell cannot always spare. When cells need to build microtubules in a matter of seconds, they instead rely on a microtubule nucleation complex called the γ-TuRC. In human cells, γ-TuRCs are anchored at microtubule organizing centers such as centrosomes, where tubulin dimers can assemble onto the γ-TuRC and rapidly polymerize into microtubules.
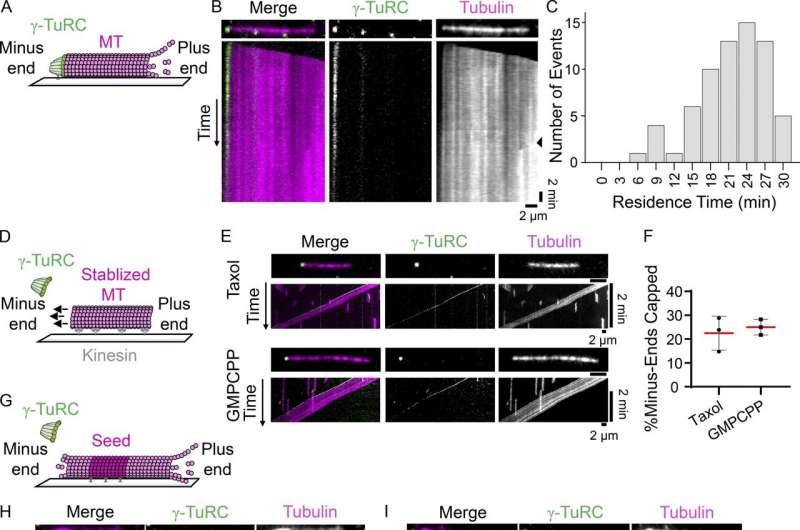
This is not, however, the only role for the γ-TuRC in microtubule formation. Studies have shown that the γ-TuRC also serves as a cap for microtubules, preventing the sudden addition or loss of tubulin dimers and ensuring that microtubules-in-action are localized to the right parts of the cell.
"Capping is another critical function of the γ-TuRC," Berman explains. "It stabilizes the microtubule, which protects it from depolymerization, and it also allows the microtubule to become anchored at specific sites, which ensures that microtubules are positioned correctly."
Kapoor, Berman, and colleagues wanted to study the γ-TuRC's capping activity in isolation, so they collaborated with the laboratory of Brian Chait to manufacture and characterize a crippled form of γ-TuRC. This mutant was incapable of nucleating microtubules but it remained to be determined how this mutation affected the γ-TuRC's capping activity.
A cap in isolation
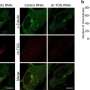
To find out whether γ-TuRC would deliver on its capping potential—or whether its nucleation function was so closely linked to its capping function that, if one went offline, the other would follow—they conducted a series of experiments and used a variety of imaging techniques to visualize the mutant γ-TuRC interacting with microtubules in vitro and in human cells.
Their results suggest that the mutant γ-TuRC can still cap microtubules—demonstrating, for the first time, that the γ-TuRC's role in capping microtubules is independent of its role in nucleating them. The team also showed that the mutant γ-TuRC plays an important role in microtubule formation outside of the centrosome during mitosis, suggesting that capping itself contributes to microtubule formation.
The findings may have long-term implications for researchers studying developmental diseases linked to γ-tubulin irregularities, such as microcephaly, and cancers including medulloblastoma, myelomas, non-small cell carcinoma, breast cancer, gliomas, and glioblastoma. The work may also fill in the blanks for scientists who have long contended with an incomplete understanding of the γ-TuRC.
For instance, Berman says, the findings are among the first to suggest that perhaps the cell can modulate between two states, choosing if the γ-TuRC should be nucleating or capping a microtubule in a context dependent manner.
"This work, which combines biochemistry, structural biology, and cell biology, is shedding light on fundamental mechanisms," Kapoor says. "In the long term, this may help us better understand the emergence of diseases related to this complex."
More information: Adi Y. Berman et al, A nucleotide binding–independent role for γ-tubulin in microtubule capping and cell division, Journal of Cell Biology (2023). DOI: 10.1083/jcb.202204102
Journal information: Journal of Cell Biology
Provided by Rockefeller University