This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
First experimental evidence showing correlation between molecular fluctuation and ligand binding
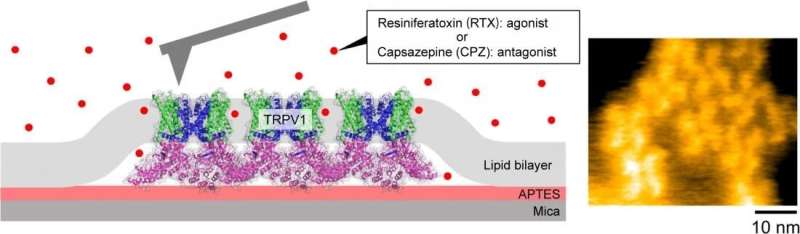
Researchers at Kanazawa University report in the Proceedings of the National Academy of Sciences high-speed atomic force microscopy experiments that show how ligands associated with stimulating and suppressing activation of the TRPV1 protein increase and decrease the molecule's structural variations. The observations provide insights into how these heat- and chili-sensing proteins function.
The skin senses heat—both from increased temperature and molecules like capsaicin in chilies—through the activation of protein receptors called Transient receptor potential vanilloid member 1 (TRPV1). However, the mechanisms behind the function of TRPV1 have not been clear. Now Ayumi Sumino at Kanazawa University in Japan and Motoyuki Hattori at the Fudan University in China and their colleagues provide important insights into this mechanism.
Using high-speed atomic force microscopy to compare the protein with and without stimulating or suppressing molecules—ligands—bound to it, they obtain what they describe as "the first experimental evidence showing the correlation between molecular fluctuation and the gating state (ligand binding)."
Once activated, the TRPV1 channel opens, allowing ions to permeate and signaling to the nervous system that a noxious stimulant is present. In 2011 researchers at the Howard Hughes Medical Institute in the U.S. put forward a theoretical basis for the activation of the receptor derived from thermodynamics, a theoretical framework that has since been corroborated by experiment.
The idea was that the molecule would respond to heat with a change in heat capacity, which is related to the fluctuations in the molecule's conformation. Structures for the TRPV1 protein were known from previous cryo electron microscopy studies but these did not clarify how the fluctuations in protein conformation might change with stimulating or suppressing molecules, or even whether temperature and chili sensing shared the same molecular mechanism.
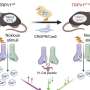
Atomic force microscopy (AFM) senses the topology of surfaces through the effect of distance on the forces on a nanosized tip positioned directly above the surface. The microscope was first invented in 1986 but was revived through work at Kanazawa University that enabled it to capture topologies at high speed thereby providing a window into the dynamics of structures.
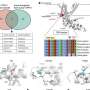
Sumino, Hattori and colleagues used high-speed AFM to image the TRPV1 receptor both in its unbound state and when bound to ligand molecules that either stimulate (agonist) or suppress (antagonist) the protein's activity. They used the molecule resiniferatoxin, which is 1,000 times hotter than capsaicin, as the agonist and for the antagonist they used capsazepine, which blocks the pain of capsaicin.
From the structures captured the researchers were able to observe fluctuations in the conformation of both the bound and unbound states of TRPV1. They found that resiniferatoxin increases conformational fluctuations, while capsazepine suppresses them.
Although the conformational fluctuations were very small—at around an Angstrom—the researchers highlight evidence in the literature of conformational changes at this scale being sufficient to affect the ion permeability of a channel. In their report of the work, the researchers conclude, "Overall, this study suggests the importance of structural fluctuation, which would be a key factor for the heat-sensing of TRPV1."
More information: Ayumi Sumino et al, Antithetic effects of agonists and antagonists on the structural fluctuations of TRPV1 channel, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2301013120
Journal information: Proceedings of the National Academy of Sciences
Provided by Kanazawa University