This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Studying battery cycling on the beamline

During his Ph.D. with TUoS, ISIS facility development student Innes McClelland developed a cell for testing battery materials during their operation using muon spectroscopy and used it to study an increasingly vital cathode material.
Understanding what is happening inside a battery material while it is charging and discharging is crucial to improving the performance of existing batteries and developing new materials for use in the batteries of the future.
One cathode material that is proving increasingly vital for future batteries is LiNi0.8Mn0.1Co0.1O2, known as NMC811. This material has a high capacity, but often suffers an irreversible capacity loss between the first charge and discharge. It's thought that this loss of capacity may be due to kinetic barriers to diffusion of the lithium ions in the material. Understanding this issue could lead to insights that inform the design of new and improved alternatives.
Muon spectroscopy is an excellent tool for studying these materials because it can probe the diffusion of ions such as lithium and sodium on a local scale, largely avoiding interfacial or grain boundary effects. Previous muon experiments on battery materials have studied the components individually, outside a battery. Although these are useful for understanding the fundamental properties, they lack an insight into the behavior of the materials during operation.
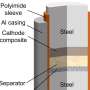
As part of his ISIS facility development studentship, Dr. Innes McClelland designed a cell that would be able to do just that. Working in collaboration with his co-supervisor beamline scientist Peter Baker and engineers from ISIS, his supervisor Professor Serena Cussen at the University of Sheffield and colleagues from the Faraday Institution next-generation cathode project FutureCat, he was able to design a cell for doing operando muon spectroscopy measurements.
As explained in their recent paper, published in Chemistry of Materials, the group were able to use this cell to study NMC811 to investigate what might be causing its lack of repeat cyclability. Using the new setup, they could measure the lithium diffusion characteristics within the material at over 70 points during the first cycle. They found that the lithium diffusion was, as expected, faster at a higher state of charge, but that it never restored to the same value as in the pristine sample.
Interestingly, by combining the differing measurement properties of muon spectroscopy with electrochemical methods, they were able to see that this slow diffusion was more prevalent on the surface of the cathode, rather than in the bulk material. This suggests that processes that focus on stabilizing the surface of the material are likely to be more successful at improving its properties.
"The exciting development of operando muon spectroscopy opens up a wide range of opportunities for researchers working on energy storage materials, allowing a unique perspective of ionic diffusion from inside the materials themselves whilst in operation," Innes explains.
He adds, "I look forward to seeing future studies which can develop the field towards a variety of battery chemistries."
More information: Innes McClelland et al, Direct Observation of Dynamic Lithium Diffusion Behavior in Nickel-Rich, LiNi0.8Mn0.1Co0.1O2 (NMC811) Cathodes Using Operando Muon Spectroscopy, Chemistry of Materials (2023). DOI: 10.1021/acs.chemmater.2c03834
Journal information: Chemistry of Materials
Provided by University of Sheffield