This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists increase efficiency of enzyme that breaks down PET plastic
One of the most widely used plastics in the world is polyethylene terephthalate, or PET for short. It is everywhere in our daily lives in the form of reusable PET drink bottles. At the end of the lifecycle of a product containing PET, the environmentally friendly reuse of the PET components through the activity of enzymes is an economically and ecologically interesting alternative to incineration, landfill or purely chemical recycling.
A team of researchers from Leipzig University has discovered how an enzyme that can break down PET works, and has further increased the efficiency of this biocatalyst. The scientists report their findings in the current issue of the journal Nature Communications.
"Our previous article on the discovery of this enzyme in the summer of 2021 already made big waves," says Dr. Christian Sonnendecker, who played a key role in the first publication. "This outstanding teamwork became the most successful research article to date in the journal ChemSusChem."
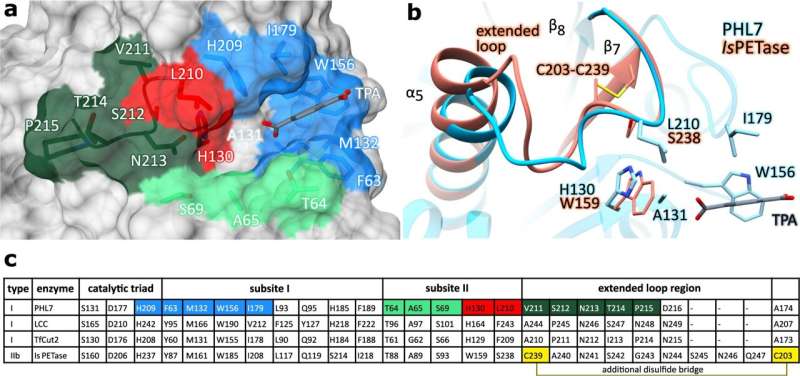
To understand how the biocatalyst works, lead author Konstantin Richter first used crystals to elucidate the spatial structure of the enzyme in his doctoral thesis. "In a way, we were following up on the determination of the first structure of a PET-degrading enzyme," says Professor Norbert Sträter, who heads the crystallographic investigations. "That was almost 10 years ago, when Wolfgang Zimmermann established this biotechnological enzyme research in Leipzig. At that time, hardly anyone had it on their radar."
To extract the secrets of PHL7's highly efficient reaction acceleration from the static crystal structures, Christian Sonnendecker enlisted the help of other experts in his research. The working groups led by Georg Künze and Christian Wiebeler used computer simulations of protein dynamics as well as quantum chemical calculations to understand the reaction mechanism and, in particular, the contribution of individual amino acids to the binding of the PET polymer, and to design better enzymes. "These predictions and calculations are extremely helpful in rationally improving an enzyme," explains Sonnendecker, "but in the end, of course, the experiment decides."
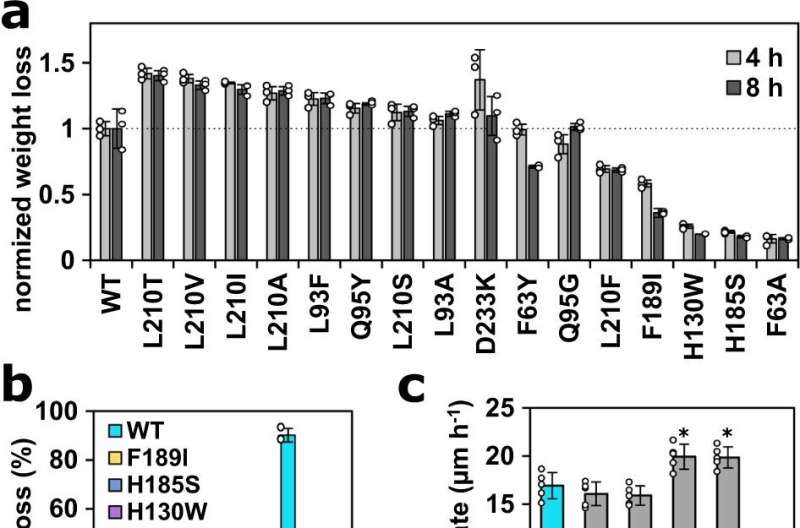
There was considerable agreement between the experimental data and the theoretical calculations. "We realized the proposed changes to the enzyme by genetic engineering and were able to further increase both its activity and its stability, which is enormously important for technical applications." Too strong binding of the enzyme to the polymeric plastic substrate would be counterproductive, the biochemist explains with regard to the proposed sliding mechanism, according to which a binding channel leads the substrate to the active center. "Sometimes less is more," says Sonnendecker.
When asked what the future holds for the research, Sonnendecker explains his plans for the interdisciplinary research network: "Together with the expert Jörg Matysik, we want to use newly developed methods of nuclear resonance spectroscopy to investigate the binding of the enzyme to the polymer substrate. This will bring our experiments closer than ever to the real processes of protein-plastic interaction."
Work is already underway on the third generation of the enzyme, extending human rational design to include machine prediction using artificial intelligence. "For this, we have completely new screening methods at our disposal, such as the so-called impedance spectroscopy platform recently developed by Ronny Frank, which feeds high-quality training data to the AI," Sonnendecker explains.
However, the early career researcher from the Institute of Analytical Chemistry at the Leipzig University sees the future primarily in bioplastics, which are based on renewable raw materials instead of petroleum-based ones and are also more readily biodegradable from the outset. A company is being set up to make his vision a reality.
"In the medium term, we are establishing a technological alternative to the fossil-dominated plastics industry and creating artificial CO2 storage," says Sonnendecker, who sees a "green future with a view to plant-based raw materials."
More information: P. Konstantin Richter et al, Structure and function of the metagenomic plastic-degrading polyester hydrolase PHL7 bound to its product, Nature Communications (2023). DOI: 10.1038/s41467-023-37415-x
Journal information: Nature Communications
Provided by Leipzig University