This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers trace genetic agent in life-threatening fungal disease
Nature has an ingenious way of taking advantage of beneficial situations.
Take Candida auris, for example. This yeast was unknown as recently as 2009, but it burst onto the scene when scientists learned it was causing life-threatening invasive infections to patients in hospitals and nursing-care facilities. In 2019, the danger from C. auris was grave enough that the U.S. Centers for Disease Control and Prevention named it a serious global health threat, citing the yeast's spread and its resistance to a host of anti-fungal drugs.
Last month, the CDC reported C. auris has been detected in nearly half the states in the U.S.
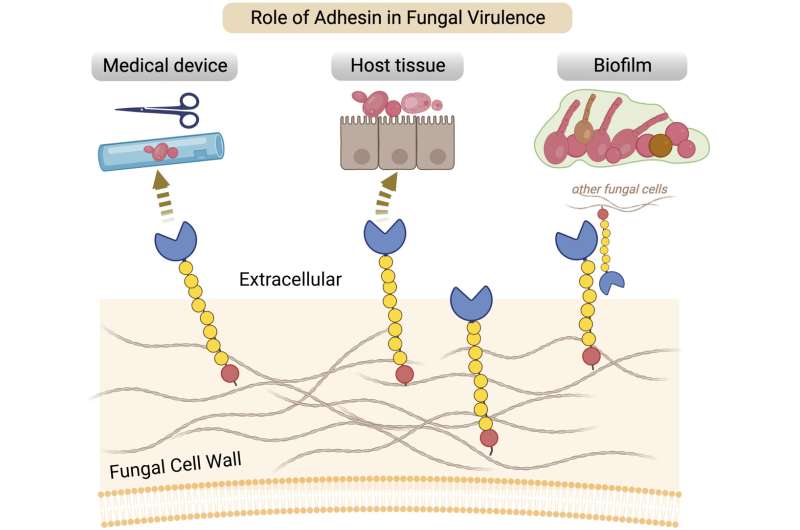
So, how did a simple, unknown yeast suddenly become a public health enemy? Part of the answer, say biologists at the University of Iowa, is found in a gene family that encodes sticky properties, or adhesins, that appear central to the virulence in fungal diseases, including some that threaten humans.
In a new study, the researchers report one such adhesin family, called the Hil family, existed in the common ancestor of all yeast species, but is more numerous in disease-causing species than benign ones. Moreover, the researchers found, some disease-causing species with a large Hil gene family are distantly related, suggesting that each species with a large Hil family independently evolved the family size, rather than having the genes passed down.
"We found this gene family has specifically and repeatedly expanded by gene duplications in pathogenic yeast lineages," says Bin He, assistant professor in the Department of Biology and a co-corresponding author on the study. "Moreover, their sequences evolved rapidly after the duplications, possibly generating functional diversity to allow the yeast to adapt to the complex host environment."
The adaptation element is key: The Hil genes likely encode proteins that allow the organism to become adhesive. More specifically, the proteins, through their structure, help yeast cells to stick to host tissue and inanimate surfaces (such as catheters), and to stitch themselves together, like interlocking Legos, to form a nearly impenetrable, drug-resistant wall, called biofilm.
It's natural selection at its finest, or most devilish, one could say. The Hil genes either are not present or not active in a bunch of other yeast species, such as baker's yeast, that are in fact beneficial to humans (assuming people like bread). But in species that are pathogenic, the researchers found, the Hil (short for the Hyr/Iff-like) family is very much alive and well, wreaking its adhesive havoc.
"That's convergent evolution," He says. "You find a way to succeed in an environmental niche."
The researchers sequenced the proteins in the adhesin family, and searched all other organisms—including the plant, animal, and bacterial kingdoms—to find out whether any other species had a similar protein sequence. They found the Hil gene family in just one place, the Saccharomycetes class, part of the Fungi kingdom.
The analysis revealed another important clue: The Hil family popped up in species that had no close relatives, taxonomically speaking. For instance, the Hil family is present, and active, in C. auris and another disease-causing species, Candida albicans. But when the researchers looked at more closely related species for each, the Hil gene number was either low, or it didn't exist at all.
"That's the idea of a parallel, or independent, evolution," He says. "Basically, these genes reached the same end state, not by descending, not by inheritance, but by independent evolution. They all took similar evolutionary paths."
The study itself sprang from a graduate-level bioinformatics class at Iowa. In fall 2019, the course's instructors centered the curriculum on C. auris, whose 5,000-gene genome had recently been sequenced. One student group decided to investigate C. auris' penchant for stickiness.
It was a wise, and fruitful, selection.
"We picked proteins based on domains with keywords that we thought could be involved in making a fungal pathogen sticky and came up with this group of adhesins," explains Lindsey Snyder, who was a student in the class and is pursuing a doctorate in genetics at Iowa. "At the time, there were two small adhesin families reported in the genome we were working with, so once we realized how large this (Hil) family was, we were pretty sure we found something that hadn't been characterized yet in this species."
Jan Fassler, a professor in the Department of Biology who conceived the class in 2013 with fellow biology associate professor Albert Erives, says the instructors would choose genomes that were new in the literature and had intriguing biological attributes.
"We chose recently sequenced genomes so that there would be very little prior investigation, thus allowing students to feel as if they were (and they were) making new discoveries," says Fassler, director of the biomedical sciences program and the co-corresponding author on the study.
The researchers next want to investigate, through experiments, specifically how the Hil family allows C. auris to become adhesive. This would advance the research beyond identifying the genes involved and could lead to medical advances.
"Here is the hope: We have identified a gene family that may play an important role in the pathogenesis and is restricted to this group of fungi. This could be a drug target if we can figure out how to inhibit it," He says.
The study, "Parallel expansion and divergence of an adhesin family in pathogenic yeasts," was published in the journal Genetics.
More information: Rachel A Smoak et al, Parallel expansion and divergence of an adhesin family in pathogenic yeasts, Genetics (2023). DOI: 10.1093/genetics/iyad024
Journal information: Genetics
Provided by University of Iowa