This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers discover how food-poisoning bacteria infect the intestines
Researchers at UT Southwestern Medical Center have discovered how a bacterium that infects people after they eat raw or undercooked shellfish creates syringe-like structures to inject its toxins into intestinal cells. The findings, published in Nature Communications, could lead to new ways to treat food poisoning caused by Vibrio parahaemolyticus.
"We have provided the first visual evidence of how a gut bacterial pathogen uses this assembly method to build a syringe to deliver a lethal injection to intestinal cells," said Kim Orth, Ph.D., Professor of Molecular Biology and Biochemistry, a Howard Hughes Medical Institute Investigator, and a W.W. Caruth, Jr. Scholar in Biomedical Research at UTSW. "This work provides a new view of how enteric bacteria when exposed to bile acids efficiently respond and build a virulence system."
V. parahaemolyticus, commonly found in warm coastal waters, is a leading cause of seafood-related food poisoning. People infected often have diarrhea, cramping, vomiting, fever, and chills.
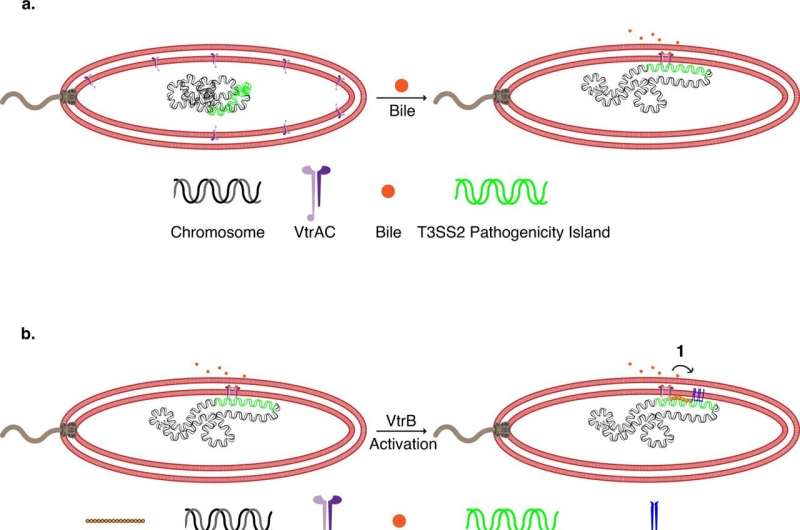
Researchers knew that V. parahaemolyticus injects molecules into human cells using a structure called the type III secretion system 2 (T3SS2). However, these syringes, composed of 19 different proteins, are not produced or assembled until the bacteria are inside the intestines. Scientists were not sure exactly how this occurs.
The latest findings build on the work of a previous study by the Orth lab. Dr. Orth and her colleagues tagged components of the V. parahaemolyticus T3SS2 with fluorescent markers and used super-resolution microscopy to track their locations as the bacteria were grown in different conditions. The researchers discovered that when V. parahaemolyticus is exposed to bile acids—digestive molecules in the intestines—the bacteria move DNA containing the T3SS2 genes close to their membrane.
Then, at the exact site where the T3SS2 is needed, V. parahaemolyticus transcribes that DNA into RNA, translates the RNA into protein, and assembles the components of the T3SS2 through the membrane in a process known as transertion. "It is like watching the assembly of a factory that produces a large molecular machine within an hour," Dr. Orth said.
These steps were previously thought to occur in more disparate locations around a cell, but pulling the machinery together into one place on the bacterium's membrane likely helps V. parahaemolyticus more quickly and efficiently build the T3SS2 and infect cells. Since other disease-causing gut bacteria contain molecular components similar to V. parahaemolyticus, the phenomenon of transertion may be widely used, the researchers hypothesize.
"Our findings imply that other gastrointestinal pathogens may also use this mechanism to mediate efficient assembly of complex molecular machines in response to environmental cues," said UTSW research specialist Karan Kaval, Ph.D., first author of the paper.
More work is needed to know which bacteria use transertion to build their T3SS structures and whether drugs could be developed that block transertion to treat V. parahaemolyticus infections.
UTSW researcher Jananee Jaishankar also contributed to this study.
More information: Karan Gautam Kaval et al, Membrane-localized expression, production and assembly of Vibrio parahaemolyticus T3SS2 provides evidence for transertion, Nature Communications (2023). DOI: 10.1038/s41467-023-36762-z
Journal information: Nature Communications
Provided by UT Southwestern Medical Center