April 19, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Broadening the scope of carbonyl olefination reactions using a new electrophotocatalytic approach
A pair of chemists at Cornell University has extended the scope of carbonyl olefination reactions by developing a new electrophotocatalytic process. In their paper published in the journal Science Advances, Keri Steiniger and Tristan Lambert describe their new process and the situations in which it might be used.
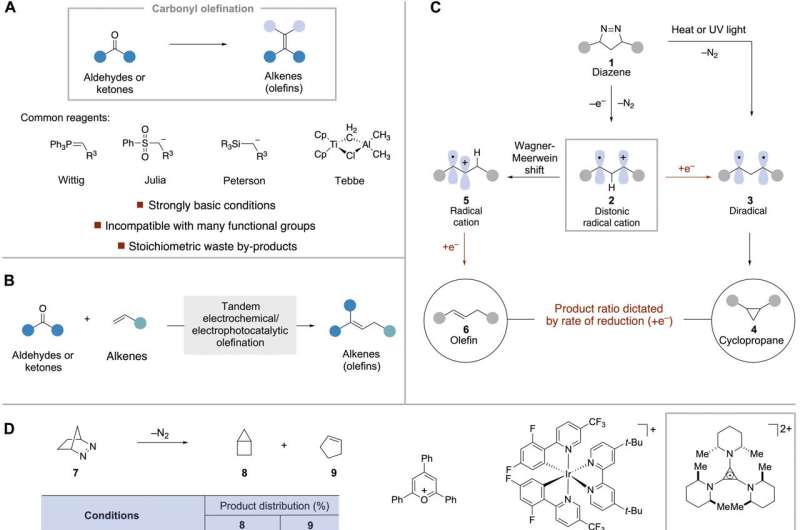
Olefins (a class of unsaturated, open-chain hydrocarbons) are often used as the basis for organic synthesis in the manufacture of plastics, the processing of petrochemicals and preparation of many types of rubbers—but they have a drawback: They generally involve use of a strong base, which tends to limit tolerance of functional groups during reactions.
In this new effort, the researchers used electrophotocatalysis to extend the scope of such reactions. In their process, electrophotocatalytic and electrochemical oxidations are paired to create intermediates in a way that can be controlled. It also allows alkenes, which serve as olefination agents, to react with both ketones and aldehydes. As the reaction occurs, two main byproducts are released—hydrogen and nitrogen gases. They acknowledge that the process looks a bit unorthodox when viewed from a distance, but insist that in practice, it works quite well.
In the new process, a diazo compound is generated electrochemically from an organic carbonyl (any of a functional group made up of a carbon atom double-bonded to an oxygen atom) along with a hydrazine (a volatile alkaline liquid). Due to cycloaddition, a cyclic diazene is produced along with the desired alkene.
As nitrogen is lost, a distonic radical cation reduction leaves the target olefin. The researchers note that the reaction allows for controlling the rate at which the electrons are transferred, which in turn allows for the conversion to an olefin, as opposed to a cyclopropane. They also note that the process can be used with a broad range of unactivated alkenes.
In addition to broadening the scope of carbonyl olefination reactions, the new process also allows for creating olefins without production of wastes typically associated with their syntheses. The research team suggests it could also be used as an alternative to Witting olefination, allowing the creation of a host of new products, including some in the pharmaceutical industry.
More information: Keri A. Steiniger et al, Olefination of carbonyls with alkenes enabled by electrophotocatalytic generation of distonic radical cations, Science Advances (2023). DOI: 10.1126/sciadv.adg3026
Journal information: Science Advances
© 2023 Science X Network