April 17, 2020 report
Using light to extend the scope of carbonylation reactions
A team of researchers at McGill University has found that light can be used to extend the scope of carbonylation reactions. In their paper published in the journal Science, the group describes using ordinary light to both break and make carbon-halogen bonds. In a Perspective piece printed in the same journal issue, Prasad Kathe and Ivana Fleischer with the University of Tübingen describe some of the problems chemists have encountered when trying to carry out carbonylation reactions, and how the work by the team in Canada solves many of the issues.
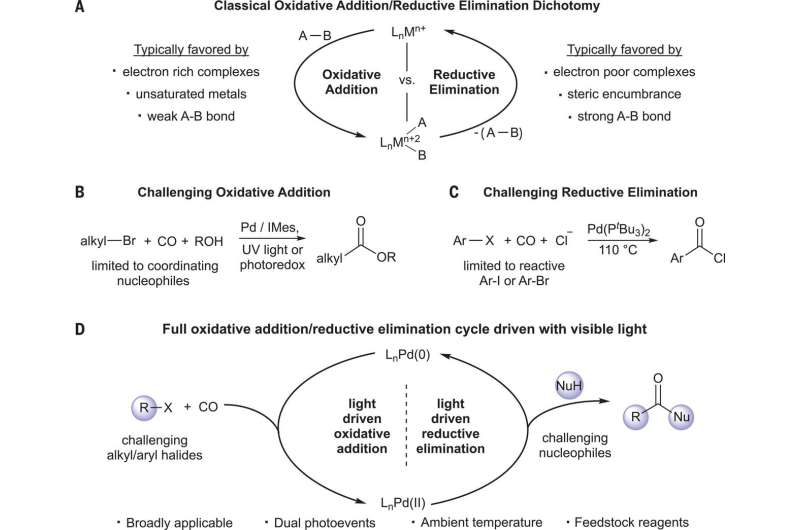
Kathe and Fleischer point out that carbonyl groups are used in a host of synthetic applications, so chemists have sought ways to extend their scope. Carbonyl groups are functional groups with carbon atoms double bonded to an oxygen atom. One of the most common ways to create them is by using carbonylation reactions in which organohalides with carbon monoxide are transformed in the presence of a nucleophile. The subsequent reactions generate a carbon-heteroatom and carbon-carbon bond and use easily obtained precursors, all in a single step, using metals as catalysts. Unfortunately, the scope of such reactions is limited. In this new effort, the researchers have found a way to overcome many of those limitations by activating the catalysts using light.
Carbonylation events typically start with setting off a reaction of the metal that is used with an electrophile. That is followed by insertion of the carbon monoxide and coordination of the nucleophile. It ends with reductive elimination. The researchers with this new effort found that visible light could excite the catalytic intermediaries and assist with reductive elimination. This allowed for a reaction between two substrates at room temperatures that are generally considered to be very challenging. This was possible because the addition of light changed the reaction from a two-electron redox event to a single-electron transfer—one that produced a radical species.
Kathe and Fleischer suggest that the discovery that light can be used to extend the scope of carbonylation reactions will likely lead to its broader use in other reactions.
More information: Gerardo M. Torres et al. A dual light-driven palladium catalyst: Breaking the barriers in carbonylation reactions, Science (2020). DOI: 10.1126/science.aba5901
Journal information: Science
© 2020 Science X Network