This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Scientists detect dimer product ROOR generated by self-reaction of ethyl peroxy radicals
Organic peroxy radicals (RO2) are important intermediates in the degradation of atmospheric volatile organic compounds. It not only participates in the cycling of atmospheric radicals and influences oxidizing capacity of the atmosphere, but also controls the formation of secondary pollutants.
Under low NOx conditions, peroxy radicals react mainly with HO2 radicals, as well as with themselves, and their products tend to have low volatility and readily enter the particulate phase. However, the associated double radical reactions are complex, the chemical mechanisms are poorly understood and experimental and theoretical studies are extremely challenging.
A collaborative team led by Prof. Weijun Zhang from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences studied the self-reaction of ethyl peroxy radicals ( C2H5O2). They combined advanced vacuum ultraviolet (VUV) photoionization mass spectrometry with theoretical calculations, providing a new insight into the direct measurement of the elusive dimeric product organic peroxides (ROOR).
The results have been published in the International Journal of Molecular Sciences.
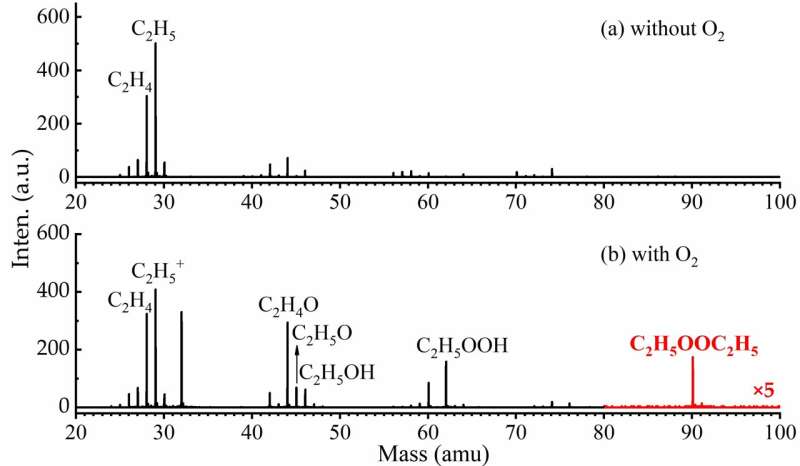
Together with scientists from the Université de Lille, France, the researchers investigated the self-reactions of C2H5O2. In addition to the main products CH3CHO, C2H5OH, C2H5O and C2H5OOH, the dimeric product C2H5OOC2H5 from the self-reaction of C2H5O2 was clearly observed for the first time in the VUV photoionization mass spectrum.
The kinetic experiments of the self-reaction of C2H5O2 and theoretical calculations were performed to verify the reaction mechanism of the ROOR product channel. The adiabatic ionization energy of C2H5OOC2H5 was also determined by measuring the synchrotron photoionization efficiency spectrum.
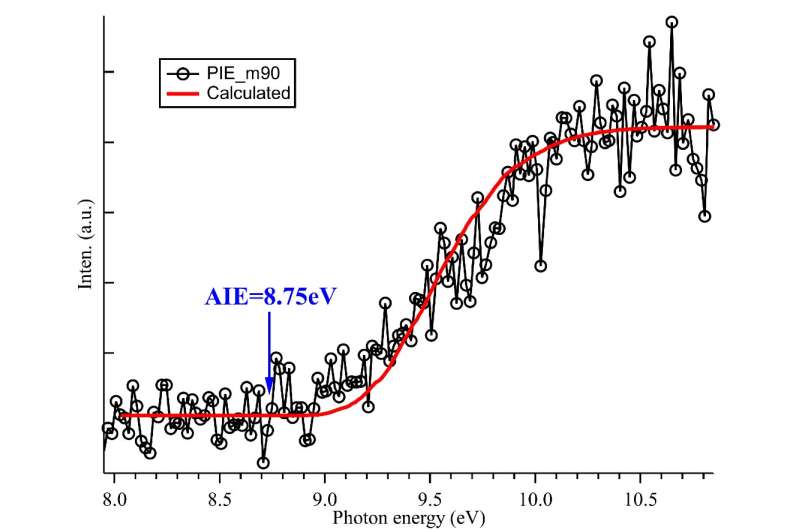
Combined with Franck-Condon factor simulations, the neutral and ionic structures of C2H5OOC2H5 were revealed.
"Our study shows that the ROOR product channel is not negligible in the small RO2 self-reactions," said Lin Xiaoxiao, a member of the team.
More information: Hao Yue et al, Dimeric Product of Peroxy Radical Self-Reaction Probed with VUV Photoionization Mass Spectrometry and Theoretical Calculations: The Case of C2H5OOC2H5, International Journal of Molecular Sciences (2023). DOI: 10.3390/ijms24043731
Provided by Chinese Academy of Sciences