This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists twist chemical bonds beyond their limits
A group of scientists from Durham University and University of York have twisted molecules to their breaking point in order to challenge the understanding of chemical bonds.
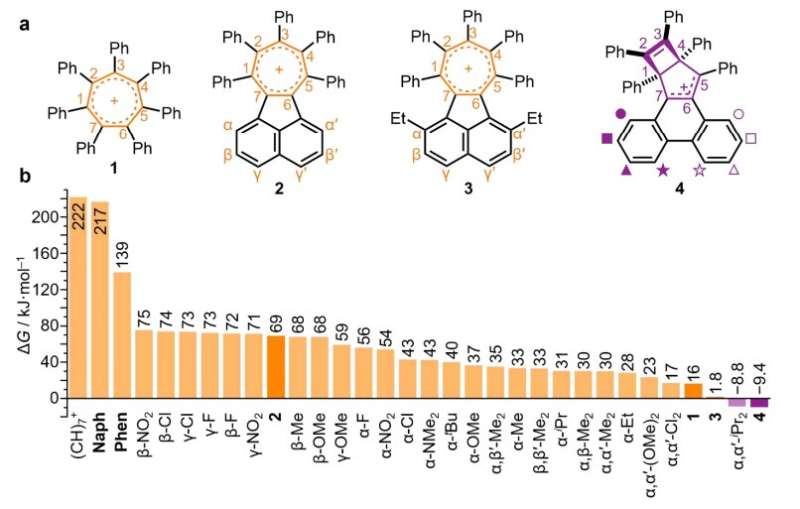
The researchers explored how far the chemical bonding in an aromatic ring can be twisted before its aromatic bonding breaks.
They achieved this by making overcrowded aromatic rings. Rather than benzene, they used tropylium, which shares electrons around a ring of seven carbon atoms.
Each of these carbon atoms can be functionalized, and having seven attachment points in the ring, rather than the six carbon atoms of benzene, allowed the researchers to cram more groups around the edge of the aromatic ring, causing more strain.
The researchers found that low levels of overcrowding made the ring twist, but without breaking its aromatic bonding.
By adding progressively larger groups around the edge of the ring, the team twisted the ring further, eventually causing the aromatic bonding to break.
The electrons no longer circle the seven carbon atoms and instead, the ring pinches across its middle to form two smaller flat rings.
Surprisingly, the researchers found there is a balance point, where the ring jumps back and forth between aromatic structure and the two smaller rings. One molecule made in this study spends 90% of its time as the pinched structure and 10% of its time as a larger aromatic ring.
Full study results have been published in the journal Nature Chemistry.
Reflecting on the study results, Dr. Paul McGonigal of University of York, said, "In these overcrowded molecules, strain and aromatic bonding are delicately balanced. The structure, properties, and potential applications of a material are ultimately determined by this balance.
"The precise control over the twisting of our molecules is unprecedented.
"We were not only able to twist an aromatic molecule up to the maximum amount of strain it can tolerate, but also to discover what happens when we push beyond that limit. We hope this investigation is a step towards us being able to more routinely turn aromatic bonding 'off' and 'on' in a controlled manner."
Project lead investigator, Promeet Saha of Durham University, said, "The reversible pinching and reopening of an aromatic ring are truly remarkable.
"Aromatic bonding is such a powerful stabilizing force that we usually think of it being a constant presence. However, our findings demonstrate that it can be surprisingly dynamic."
Chemical bonding in aromatic molecules is key to the structure, stability and function of chemicals such as drugs and plastics.
More information: Paul McGonigal, Rupturing aromaticity by periphery overcrowding, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01149-6. www.nature.com/articles/s41557-023-01149-6
Journal information: Nature Chemistry
Provided by Durham University